What is the rate of the reaction?
- Rate of reaction measures the speed at which the reactants are converted to the products in a chemical reaction.
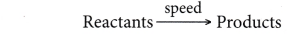
- For a reaction that occurs rapidly, the rate of reaction is high. Conversely, for a reaction that occurs slowly, the rate of reaction is low.
- The time taken for a fast reaction is short, whereas the time taken for a slow reaction is long.
- Hence, the rate of a particular reaction is inversely proportional to the time taken for the reaction.
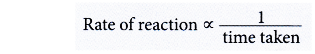
- Different chemical reactions occur at different rates. Some examples are illustrated in Table.
| Type of reaction | Fast reaction | Slow reaction |
| Reaction involving liberation of a gas | Bubbles of carbon dioxide gas liberate rapidly when sodium carbonate powder reacts with dilute hydrochloric acid. Na2CO3(s) + 2HCl(aq) → 2NaCl(aq) + CO2(g) + H2O(l) | In photosynthesis, carbon dioxide reacts with water very slowly in the presence of sunlight and chlorophyll to produce glucose and oxygen gas. 6CO2(g) + 6H2O(l) → C6H12O6(s) + 6O2(g) |
| Precipitation reaction | When silver nitrate solution is added to sodium chloride solution, a white precipitate of silver chloride is formed immediately. AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) | When dilute hydrochloric acid is added to sodium thiosulphate solution, a yellow precipitate of sulphur appears only after a few seconds. |
| Heating a metal in air | When a small piece of potassium is heated in air, it burns rapidly to form a white solid of potassium oxide. 4K(s) + O2(g) → 2K2O(s) | When a small piece of copper is heated in air, it reacts slowly with oxygen in the air to form a black solid of copper(II) oxide. |
People also ask
- How do you calculate the reaction rate?
- What factors affect the rate of a reaction?
- How does the surface area affect the rate of reaction?
- Explain the effect of concentration on the rate of reaction?
- How does the temperature affect the rate of a chemical reaction?
- What is the effect of a catalyst on the rate of a reaction?
- What is the collision theory in chemistry?
- How does the collision theory affect the rate of reaction?
What does the rate of reaction measure?
Observable changes for measuring the rate of reaction:
1. When a reaction occurs, two obvious changes that occur are:
- the quantity of a reactant decreases with time
- the quantity of a product increases with time
2. The quantity of a reactant/product can be the
- number of moles of a substance
- mass of a solid
- volume of a gas
- concentration of a solution
3. If the changes of any of these quantities are visible and measurable during a reaction, then it can be used to measure the rate of that reaction.
4. Suitable measurable visible changes in a chemical reaction are:
- volume of a gas liberated
- formation of a precipitate
- changes in the mass during a reaction
- colour changes
- changes in the electrical conductivity of the solution
- temperature changes
- pressure changes
- changes in concentration of the solution of a reactant
- pH changes
5. One of these measurable visible changes can be selected as a suitable quantity to determine the rate of a particular reaction.
The changes in this selected quantity can be measured by carrying out an experiment and the results are then analysed to determine the rate of that reaction.
6. Definition: Rate of reaction is defined as the change in a selected quantity during a reaction per unit time whereby the selected quantity can be any of the measurable visible changes in the reaction.
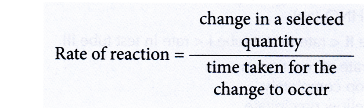
7. Two examples to illustrate the meaning of rate of reaction.
Reaction between magnesium and dilute sulphuric acid
Mg(s) + H2SO4(aq) → MgSO4(aq) + H2(g)
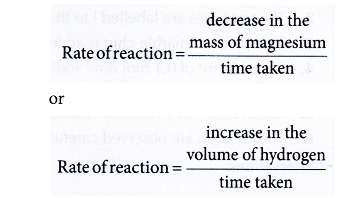
- In the reaction between dilute sulphuric acid and a magnesium ribbon, the following two changes are observed:
- The mass of magnesium (the reactant) decreases with time.
- The volume of hydrogen gas (the product) increases with time.
- Hence, the rate of reaction between dilute sulphuric acid and magnesium can be determined by measuring the change in the mass of magnesium or the volume of hydrogen gas per unit time.
Quantitatively,
- Reaction between ethanedioic (oxalic) acid, H2C2O4 and acidified potassium manganate(VII) solution.
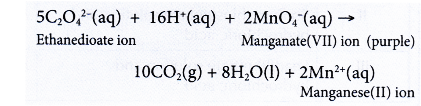
- When excess aqueous ethanedioic acid is added to acidified potassium manganate(VII) solution, the purple colour of the solution slowly decolourises at room temperature.
- By measuring the time taken for the purple colour to decolourise, the rate of reaction can be determined.
- Rate of reaction is inversely proportional to the time taken for the purple colour to decolourise.

- Rate of reaction is reflected by the value of 1/time taken. The larger the value time taken of 1/time taken, the higher the rate of reaction.
- Table shows the units for the rate of reaction measured in different ways.
Change in a selected quantity per unit time Units for the rate of reaction Change in mass per unit time g s-1 or g min-1 Change in volume of a gas liberated per unit time cm3 s-1 or cm3 min-1 Change in concentration of a reactant per unit time mol dm-3 s-1 or mol dm-3 min-1 Change in number of moles of a reactant per unit time mol s-1 or mol min-1
Rate of Reaction Experiment
Aim: To compare the rates of a few reactions.
Materials: Marble chips, 2 mol dm-3 hydrochloric acid, 0.1 mol dm-3 sodium thiosulphate solution, 1 mol dm-3 lead(ll) nitrate solution.
Apparatus: 50 cm3 beakers, test tubes.
Procedure:
- 5 cm3 of 2 mol dm-3 hydrochloric acid is poured into each of the three test tubes on a rack.
- The test tubes are labelled I to III respectively.
- One piece of marble chip is added into test tube I.
- About 2 cm3 of 0.1 mol dm-3 sodium thiosulphate solution is poured into test tube II and the mixture is shaken well.
- About 2 cm3 of 1 mol dm-3 lead(II) nitrate solution is poured into test tube III and the mixture is shaken well.
- The changes are observed carefully. The rates of reactions in the three test tubes are compared.
Observations:
| Test tube | Reactants | Observation |
| I | Marble chip and hydrochloric acid | Bubbles of a colourless gas are liberated rapidly, that is, effervescence occurs rapidly. |
| II | Sodium thiosulphate solution and hydrochloric acid | A yellow precipitate appears only after about 12 seconds. |
| III | Lead(II) nitrate solution and hydrochloric acid | A white precipitate is formed immediately. |
Inferences:
- The reaction between lead(ll) nitrate solution and hydrochloric acid is very fast.
- The reaction between the marble chip and hydrochloric acid is moderately fast.
- The reaction between sodium thiosulphate solution and hydrochloric acid is slow.
Discussion:
- The chemical equation for the reaction in
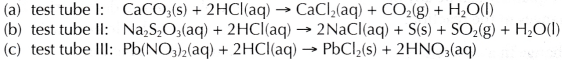
- The rate of reaction in ascending order is: rate in test tube II < rate in test tube I < rate in test tube III
- The observable change that can be used to compare the rate of reaction in
(a) test tube I is the time taken for the effervescence to stop completely.
(b) test tube II is the time taken for the appearance of a yellow precipitate.
(c) test tube III is the time taken for the appearance of a white precipitate.
Conclusion:
- The rate of reaction between sodium thiosulphate solution and hydrochloric acid is the lowest.
- The rate of reaction between the marble chip and hydrochloric acid is moderately high.
- The rate of reaction between lead(II) nitrate solution and hydrochloric acid is the highest.
