Explain the Versatile Nature of Carbon
By the eighteenth century scientists tried to explain the difference between the compounds in a broader way.
J. J. Berzelius (1807) named the compounds that derived from living organism as organic compounds and those from non-living materials as inorganic compounds. He thought that organic compounds would be prepared in the living bodies through vital force, the essence of life. As this force is absent outside the living bodies, it was thought that the so called organic compounds could not be synthesized in the laboratories.
Surprisingly F. Wohler (1828) produced an organic compound Urea in the laboratory by heating an inorganic salt ammonium cyanate.
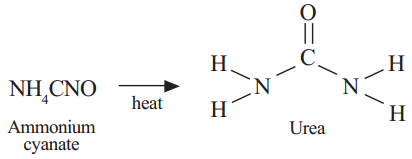 It inspired many other chemists and they were successful to prepare the so called organic compounds, methane, acetic acid etc., in the laboratory. This gave a death blow to the idea that organic compounds are derived from living organism. Chemists thought about a new definition for organic compounds. After observing the structures and elements of organic compounds, they defined organic compounds as compounds of carbon. Therefore, organic chemistry is totally allotted to carbon compounds.
It inspired many other chemists and they were successful to prepare the so called organic compounds, methane, acetic acid etc., in the laboratory. This gave a death blow to the idea that organic compounds are derived from living organism. Chemists thought about a new definition for organic compounds. After observing the structures and elements of organic compounds, they defined organic compounds as compounds of carbon. Therefore, organic chemistry is totally allotted to carbon compounds.
We understand that all molecules that make life possible carbohydrates, proteins, nucleic acids, lipids, hormones, and vitamins contain carbon. The chemical reactions that take place in living systems are of carbon compounds. Food that we get from nature, various medicines, cotton, silk and fuels like natural gas and petroleum almost all of them are carbon compounds. Synthetic fabrics, plastics, synthetic rubber are also compounds of carbon. Hence, carbon is a special element with the largest number of compounds.
Read More:
- Binding of Carbon with other Elements
- Homologous Series of Hydrocarbons
- Allotropes of Carbon
- Catenation in Carbon
- Classification of Hydrocarbons
- sp3 Hybridized Carbon atom
- Hybridization of the Carbon atoms in Acetylene
- What are the Characteristics of Compounds
- Chemical Properties of Carbon Compounds
- Nomenclature of Carbon Compounds
