Uses of Acids
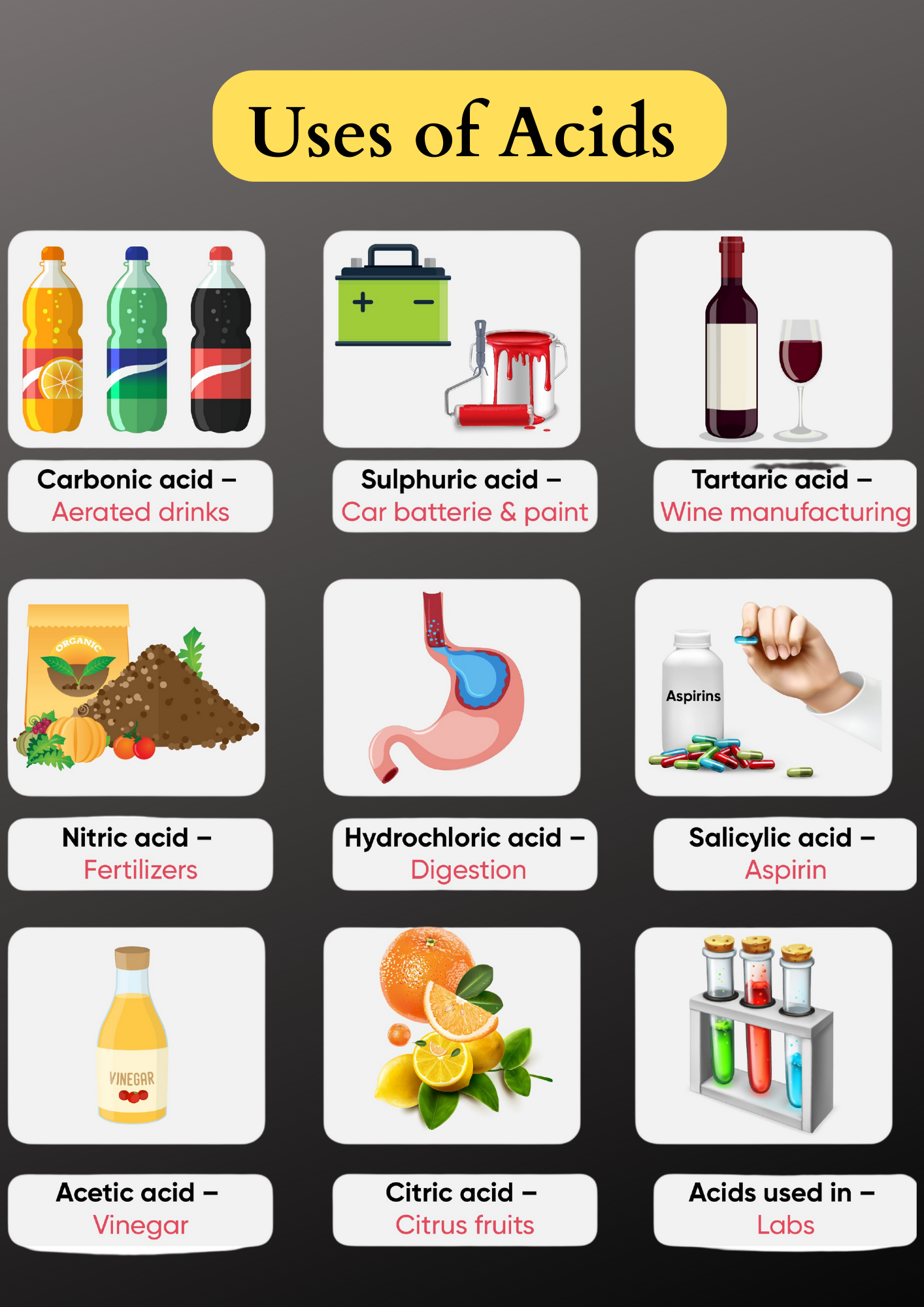
1. Hydrochloric Acid (HCl)
- Dilute hydrochloric acid is used in various industries that use heating applications. It is applied to remove deposits from the inside of the boilers.
- Hydrochloric acid is also used for cleaning sinks and sanitary ware.
2. Sulphuric Acid (H2SO4)
Sulphuric acid is such an important industrial chemical that it is called the king of chemicals. Some of its major uses are as follows:
- Sulphuric acid is used in car batteries.
- It is used in the manufacture of paints, drugs, dyes, and to produce fertilizers.
3. Nitric Acid (HNO3)
- It is used by goldsmiths for cleaning gold and silver ornaments.
- It is also used for the production of fertilizers such as ammonium nitrate.
4. Acetic Acid (CH3COOH)
- Acetic acid is used directly to enhance the flavour of food. In fact, we commonly know acetic acid as vinegar.
- It is also used as a cleansing agent in products meant for cleaning windows, floors, utensils, etc.
- It also helps to remove stains on woodwork such as furniture and carpets.
- Acetic acid is used as a preservative in pickles, etc. Most microorganisms cannot live in an acidic environment. An acidic environment either slows down their activities or can also kill them. This is why you will find vinegar in many commonly packaged food items such as pickles, sauce, ketchup, etc.
The following table shows the uses of some organic and inorganic acids.
| Acid | Uses |
| Organic acids | |
| Citric acid |
|
| Ascorbic acid (also called vitamin C) |
|
| Acetic acid |
|
| Tartaric acid |
|
| Inorganic acids | |
| Hydrochloric acid |
|
Nitric acid |
|
Sulphuric acid |
|
| Phosphric acid |
|
Boric acid |
|
| Acid | Sulphuric acid | Nitric acid | Benzoic acid | Ethanoic acid | Methanoic acid |
| Use | To make
| To make
|
|
|
|
People also ask
- What is the definition of an acid and a base?
- What is the definition of an acid in chemistry?
- What is the definition of a base in chemistry?
- Classification of Acids
- Preparation of Acids
- What are the chemical properties of an acid?
- General Properties of Acids
- Preparation of Bases
- General Properties of Bases
- What determines a Strong Base and a Weak Base
- What are the uses of Bases
- How can we measure the strength of acids and alkalis?
- How to calculate concentration of acids and alkalis?
- How do you prepare a standard solution?
- What is meant by a neutralization reaction?
- How does titration determine concentration?
- Relationship between pH values and molarity of acids and alkalis
- Concept of the pH Scale
- Role of pH in everyday life
- What is the pH of a salt solution
