What is Thermal Equilibrium?
Thermal Equilibrium
 What happens when cold milk is poured into a cup of hot coffee?
What happens when cold milk is poured into a cup of hot coffee?
The cold milk warms up while the hot coffee cools down. After some time, they are at a state known as thermal equilibrium.
At thermal equilibrium, the mixture of milk and coffee are at the same degree of hotness.
The mechanism of thermal equilibrium is shown schematically in Figure.
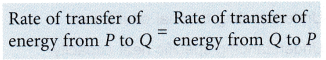 The net transfer of energy or the heat transferred = 0
The net transfer of energy or the heat transferred = 0
when P and Q are at thermal equilibrium.
People also ask
Which Instrument is Used to Measure the Temperature?
- The meaning of thermal equilibrium:
Two objects at thermal equilibrium have the same temperature and there is no net transfer of heat energy between the objects. - Two objects at different temperatures in thermal contact will eventually come to a state of thermal equilibrium regardless of the
(a) mass,
(b) size,
(c) shape,
(d) type of material of the two objects. - Good thermal contact ensures that the objects come to thermal equilibrium after a shorter period of time.
- If two objects are thermally insulated from each other, heat cannot be transferred between them. It will not be possible for them to come to thermal equilibrium.
- Before thermal equilibrium
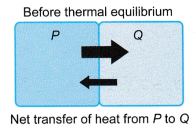
- P transfers energy at a higher rate to Q.
- Q transfers energy at a lower rate to P.
- There is a net transfer of energy from P to Q. The energy transferred is known as heat.
- P loses energy and cools down, while Q gains energy and warms up.
- At thermal equilibrium
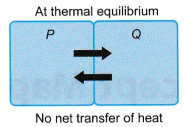
- P and Q are at the same temperature.
- P and Q transfer energy at equal rates to each other.
- Net transfer of energy between P and Q = 0.
- P and Q are at thermal equilibrium.
Examples of Thermal Equilibrium
Refrigerator
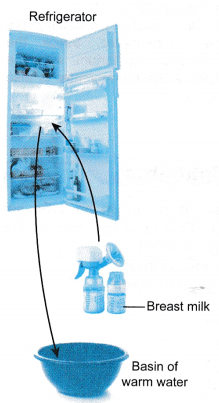
- A thermostat maintains the refrigerator at a constant low temperature.
- Heat energy is relased from the milk to the cooler surroundings in the refrigerator until thermal equilibrium is reached.
- The milk is kept fresh at a low temperature.
- Heat energy is transferred from the warm water to the cooler milk until thermal equilibrium is reached.
- The warm milk is now ready for the baby to drink.
Person with fever
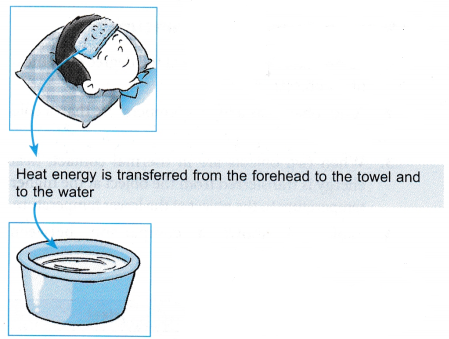
- A cool wet towel is placed on the forehead.
- Heat is transferred from the forehead to the towel until thermal equilibrium is reached.
- The warm towel is immersed and rinsed in tap water.
- Heat is transferred from the towel to the water until thermal equilibrium is reached at a lower temperature.
Clinical thermometer

- A thermometer is placed under the tongue so that there is good thermal contact.
- The doctor waits about 1 minute for the thermometer to come to thermal equilibrium with the patient.
- The reading of the thermometer is the body temperature of the patient.
Air conditioner

- The air conditioner maintains the room at a cool temperature of 24°C.
- Heat is released by the warmer objects in the room until thermal equilibrium is reached
- All the objects in the room will be at a temperature of 24°C.
Oven

- A thermostat keeps the inside of the oven at a constant high temperature.
- The food absorbs heat until thermal equilibrium is reached.
- The temperature of the food is equal to the temperature of the oven.
