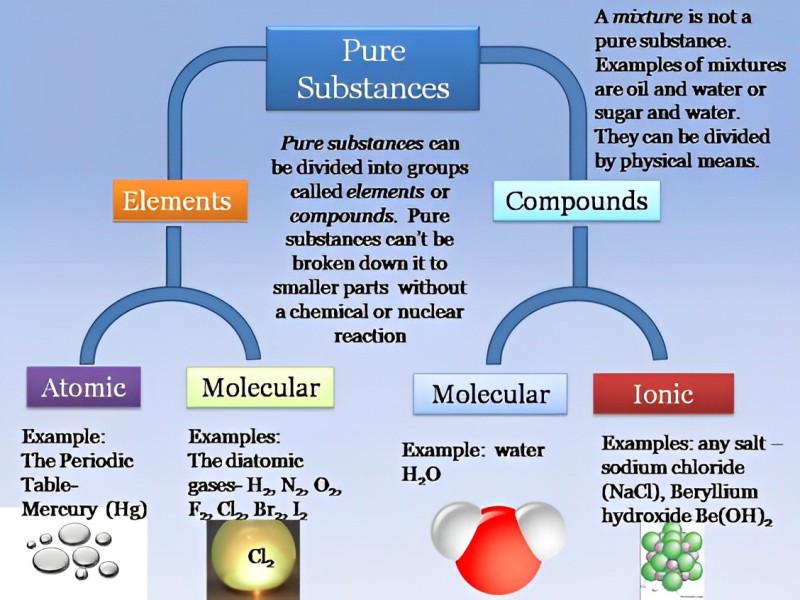
Classification Of Pure Substances
Chemical substances are made of atoms and molecules. An atom is the smallest particle of a substance. Atoms may or may not exist independently. Molecules are formed when two or more atoms join together. Molecules can exist independently. Chemical substances can be divided into two major groups: pure substances and mixtures. Pure substances can be elements or compounds.
Element
An element is a substance that is made up of only one kind of atom and cannot be broken down into simpler substances by chemical means. For example, silver, gold, aluminium, hydrogen, oxygen, etc. Each element has its own distinct set of properties, thus, the atoms of an element will have all the properties of that particular element.
Compounds
A compound is a substance that is formed when two or more elements combine chemically.
The smallest particle of a compound is a molecule which shows all the properties of that compound. However, the properties of a compound are entirely different from those of its constituent elements.
For example, when hydrogen combines with oxygen, both of which are gases, a liquid named water is formed.
Mixtures
Mixtures consist of two or more substances simply mixed, but not chemically combined.
For example, air is a mixture of various gases such as nitrogen, oxygen, carbon dioxide, water vapour, argon, and particles of dust, smoke, and many others.
A mixture retains the properties of its components, e.g., in a mixture of iron filings and sulphur, both retain their characteristic properties, i.e., iron is still attracted by a magnet and sulphur remains as a powder.
