How does titration determine concentration?

- Titration is a very useful laboratory technique in which one solution is used to analyse another solution.
- One of the solutions is a standard solution of known concentration and is delivered from a burette.
- The technique involves determining accurately the volume of the standard solution needed to react exactly with a known volume of another solution contained in a conical flask in a reaction ffor which the equation (stoichiometry) is known.
- The completion of the reaction occurs when enough standard solution is added from the burette to react exactly with the other solution in the conical flask.
- To detect the completion of the reaction, an indicator is used.
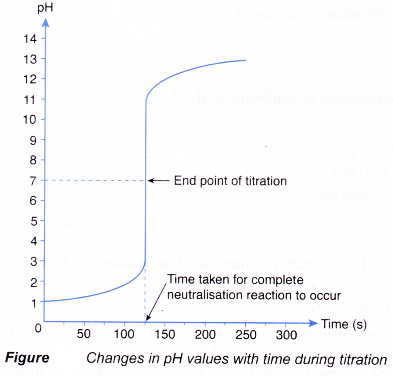
- The point at which the indicator changes colour is called the end point.
The end point is the point in the titration at which the indicator changes colour. - Table shows the common acid-base indicators and their colour changes in acidic and alkaline solutions.
Indicator Colour in acidic solution Colour in neutral solution Colour in alkaline solution Litmus solution Red Purple Blue Methyl orange Red Orange Yellow Phenolphthalein Colourless Colourless Pink Universal indicator solution Red/orange/yellow Green Purple - The end point can also be determined by using a pH meter and a conductivity cell.
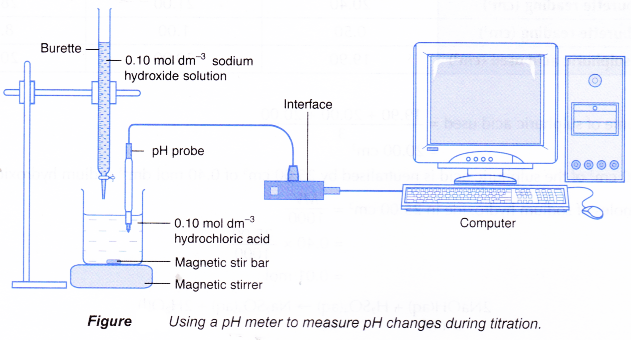
- Figure shows the set-up for a titration using a pH meter to detect the end point.
(a) The pH meter can be interfaced with a computer to allow a graph of pH against time to be plotted.
(b) The volume of alkali needed can be calculated from the reaction time and the rate the alkali is added to the acid.
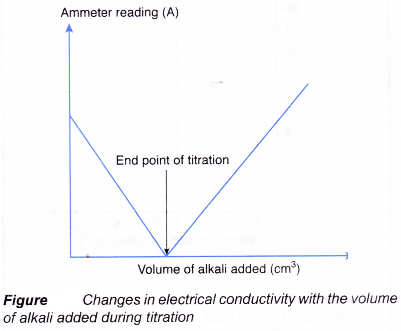
- Figure shows a set-up for a titration using a conductivity cell to detect the end point.
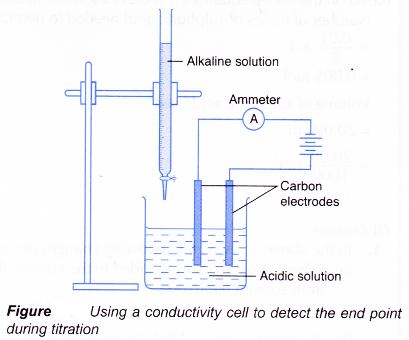
(a) Acidic and alkaline solutions conduct electricity because they contain ions.
(b) When an alkali is slowly added to an acid, the electrical conductivity of the acidic solution decreases.
(c) The hydrogen ions and hydroxide ions combine to form water molecules which cannot conduct electricity.
(d) The concentration of mobile ions in the solution decreases, making the solution less conducting.
(e) Figure shows the changes in electrical conductivity with the volume of alkali added.
People also ask
- What is the definition of an acid and a base?
- What is the definition of an acid in chemistry?
- What is the definition of a base in chemistry?
- Classification of Acids
- Preparation of Acids
- What are the chemical properties of an acid?
- General Properties of Acids
- Uses of Acids
- Preparation of Bases
- General Properties of Bases
- What determines a Strong Base and a Weak Base
- What are the uses of Bases
- How can we measure the strength of acids and alkalis?
- How to calculate concentration of acids and alkalis?
- How do you prepare a standard solution?
- What is meant by a neutralization reaction?
- Relationship between pH values and molarity of acids and alkalis
- Concept of the pH Scale
- Role of pH in everyday life
- What is the pH of a salt solution
Acid Base Titration Experiment
Aim: To determine the end point of a titration between sodium hydroxide solution and sulphuric acid and hence calculate the concentration of the sulphuric acid.
Materials: 0.40 mol dm-3 sodium hydroxide solution, sulphuric acid (about 0.2 mol dm-3), phenolphthalein
Apparatus: Burette, pipette, pipette filler, beaker, conical flask, burette stand and clamp, white tile, filter funnel
Procedures:
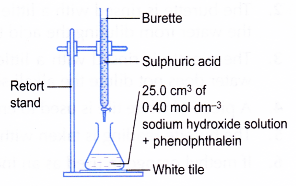
- A clean burette is rinsed with a little sulphuric acid. The burette is clamped to a retort stand.
- The burette is filled with sulphuric acid. The initial burette reading is recorded.
- A clean pipette is rinsed with sodium hydroxide solution.
- 25.0 cm3 of the aqueous sodium-hydroxide is pipetted into a conical flask.
- Two drops of phenolphthalein are added to the conical flask. The alkali solution turns pink.
- The flask is placed on top of a white tile as shown in Figure.
- The sulphuric acid is added slowly into the conical flask. The solution in the flask is swirled continuously.
- When the solution in the conical flask starts to become pale pink, the acid is added drop by drop. The conical flask is shaken after each drop of acid is added.
- The addition of the acid is stopped when the pink colour just disappears.
- The final burette reading is recorded.
- The above titration is repeated a few times to obtain accurate and consistent results.
Results:
| Titration number | 1 | 2 | 3 |
| Final burette reading (cm3) | 20.40 | 21.00 | 28.00 |
| Initial burette reading (cm3) | 0.50 | 1.00 | 8.00 |
| Volume of sulphuric acid used (cm3) | 19.90 | 20.00 | 20.00 |
Calculation:

Hence, 20.00 cm3 of the sulphuric acid is neutralised by 25.00 cm3 of 0.40 mol dm-3 sodium hydroxide solution.
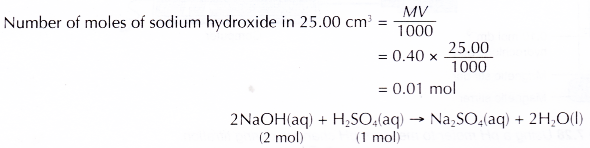
Based on the above equation, 2 moles of sodium hydroxide are needed to neutralise 1 mole of sulphuric acid Number of moles of sulphuric acid needed to neutralise 0.01 mole of sodium hydroxide.

Discussion:
- In the above acitivity, the following changes occurred:
(a) When sulphuric acid is added to the conical flask, the H+ ion from sulphuric acid neutralises the OH- ion from sodium hydroxide
H+(aq) + OH–(aq) → H2O(l)
(b) As more H+ ion is added, the neutralisation reaction decreases the concentration of OH– ion in the conical flask. Hence, the colour of the phenolphthalein indicator becomes more pale.
(c) At the end point, all the OH– ions in the conical flask are neutralised by the H+ ions. The indicator turns colourless.
(d) The products of neutralisation are sodium sulphate and water. The conical flask contains aqueous sodium sulphate. Mobile ions, Na+ and SO42- are present in the solution. - The burette is rinsed with a little sulphuric acid to remove water present inside the burette. This is to prevent the water from diluting the acid that is poured into the burette.
- The pipette is rinsed with a little sodium hydroxide solution to remove water inside the pipette so that the water does not dilute the alkali solution being suck into the pipette.
- A piece of white tile is used to enable the change in colour of the phenolphtalein indicator to be clearly seen.
- The burette reading is taken with the eye placed at the same level as the.meniscus.
- If methyl orange in used as an indicator, the colour change will be from yellow to orange.
Calculations involving neutralisation

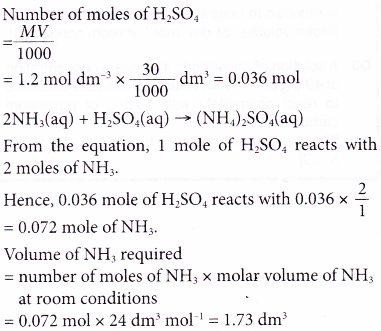
1. What volume of ammonia gas, measured at room conditions, is required to neutralise 30 cm3 of 1.2 mol dm-3 sulphuric acid?
[Molar volume: 24 dm3 mol-1 at room conditions]
Solution:
2. A student used a standard solution of sodium hydroxide to determine the concentration of a solution of hydrochloric acid.
Concentration of sodium hydroxide solution = 0.10 mol dm-3
Volume of hydrochloric acid used = 25.00 cm3
Burette reading:
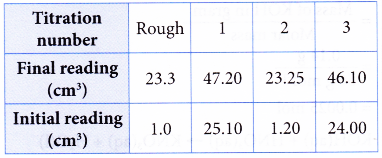
What is the molarity of the hydrochloric acid?
Solution:
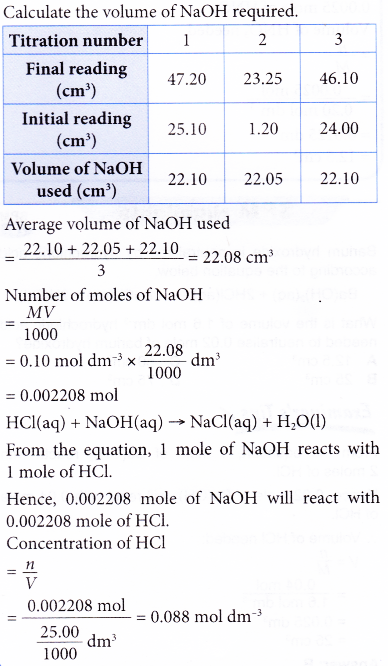
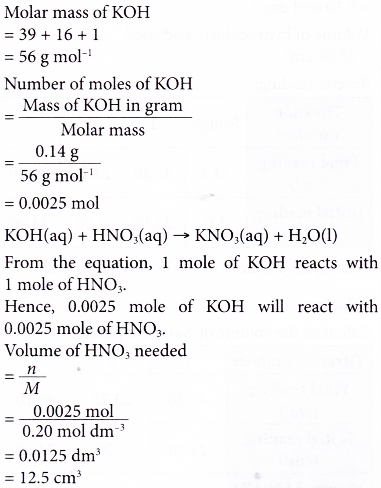
3. What volume of 0.20 mol dm-3 nitric acid is required to neutralise 0.14 g of potassium hydroxide? [Relative atomic mass: O, 16; K, 39]
Solution: