What is the Hybridization of the Carbon atoms in Ethylene
sp2 Hybridisation
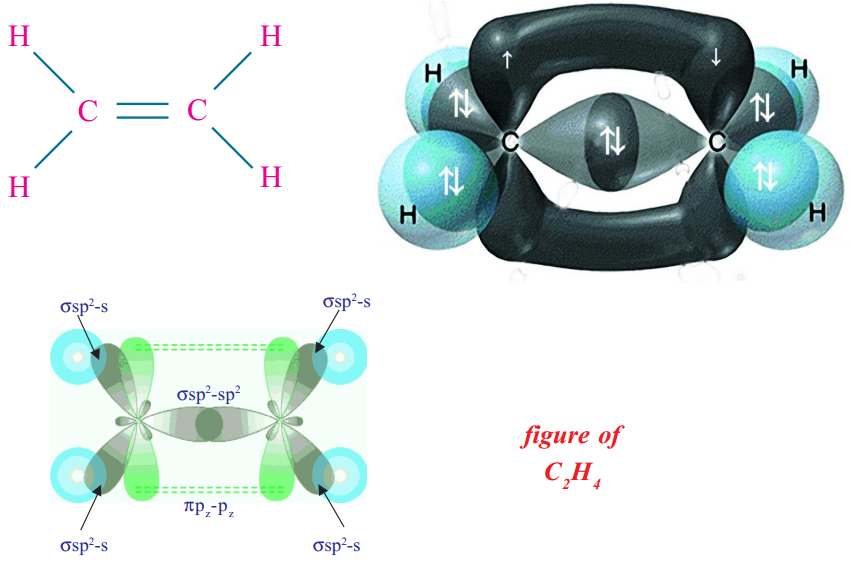
Consider ethene (ethylene, CH2 = CH2) molecule as the example.
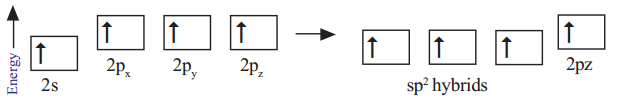
In the formation of CH2 = CH2 each carbon atom in its excited state undergoes sp2 hybridisation by intermixing one s-orbital (2s) and two p-orbitals (say 2px , 2py) and reshuffling to form three sp2 orbitals. Now each carbon atom is left with one ‘p’ orbital (say pz) unhybridised. The three sp2 orbitals having one electron each get separated around the nucleus of carbon atoms at an angle of 120o. When carbon is ready to form bonds one sp2 orbital of one carbon atom overlaps the sp2 orbital of the other carbon atom to form sp2 – sp2 sigma (σ) bond.
The remaining two sp2 orbitals of each carbon atom get overlapped by‘s’ orbitals of two hydrogen
atoms containing unpaired electrons. The unhybridised pz orbitals on the two carbon atoms overlap laterally as shown in figure to form a π bond.That means there exists a sigma (σ) bond and a pi (π) bond between two carbon atoms in ethene molecule. Hence, the molecule ethene (C2H4) is
The common name for Ethene is Ethylene.
