What is the Hybridization of the Carbon atoms in Acetylene
sp Hybridisation
Each carbon is only joining to two other atoms rather than four (as in methane or ethane) or three (as in ethene). Here the carbon atoms hybridise their outer orbitals before forming bonds, this time they only hybridise two of the orbitals.
They use the ‘s’ orbital (2s) and one of the 2p orbitals, but leave the other 2p orbitals unchanged. The new hybrid orbitals formed are called sp hybrid orbitals, because they are made by an s-orbital and a p-orbital reorganizing themselves.
To know the ability of ‘C’ to form one single bond and one triple bond, let us consider ethyne (acetylene, C2H2) molecule as our example.
In acetylene molecule there exists a triple bond between two carbon atoms and the fourth valency of each carbon atom is satisfied by hydrogen atoms (H–C ≡ C–H )
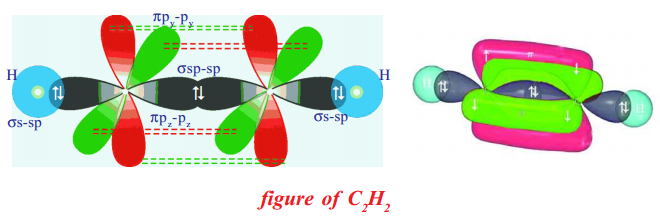
In C2H2 molecule there are two carbon atoms and two hydrogen atoms.
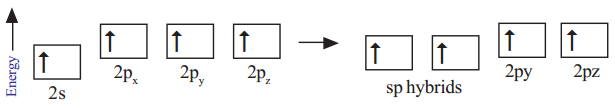
In excited state each carbon atom undergoes sp- hybridisation by mixing its one ‘s’ orbital (2s) and one ‘p’ orbital (2px) and reshuffling to form two identical orbitals known as sp-orbitals. Each carbon atom has two unhybridised p-orbitals (say 2py, 2pz).
One sp-orbital of a carbon overlaps the sp-orbital of other carbon to give sp-sp sigma bond. The other sp-orbital of each carbon atom overlaps ‘ s ’ orbital of a hydrogen atom to form a s-sp sigma bond. The unhybridised ‘p’ orbitals of one carbon atom laterally overlap the unhybridised ‘p’ orbitals of other carbon atom to give two π bonds between two carbon atoms (say πpy-py, πpz-pz , see figure). Thus ethyne molecule H–C ≡ C–H and there exists three σ-bonds and two π-bonds in the molecule.
Read More:
- Binding of Carbon with other Elements
- Homologous Series of Hydrocarbons
- Allotropes of Carbon
- Catenation in Carbon
- Classification of Hydrocarbons
- sp3 Hybridized Carbon atom
- Versatile Nature of Carbon
- What are the Characteristics of Compounds
- Chemical Properties of Carbon Compounds
- Nomenclature of Carbon Compounds
