How does the temperature affect the rate of a chemical reaction?
Effect of temperature on the rate of reaction:
- When the temperature increases, the rate of reaction also increases.
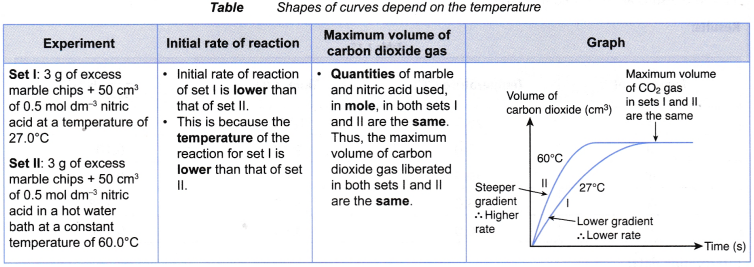
- (a) For example, two sets of experiments are carried out using the reacting conditions below:
Set I: 1 g of granulated zinc and 20 cm3 of 0.2 mol dm–3 hydrochloric acid at 60°C
Set II: 1 g of granulated zinc and 20 cm3 of 0.2 mol dm–3 hydrochloric acid at 30°C
(b) The rate of reaction of set I is higher than that of set II.
(c) This is because the temperature (60°C) of the reacting mixture in set I is higher than the temperature (30°C) of the reacting mixutre in set II. - When investigating experimentally the effect of temperature on the rate of reaction,
- the experiment is repeated a few times, each time using a different temperature of a reactant.
- all the other factors/conditions are kept constant in all the experiments.
- The following shows an example of the effect of temperature on the rate of reaction. Table shows the method to derive the shapes of curves plotted for two sets of experiments.
People also ask
- What is the rate of the reaction?
- How do you calculate the reaction rate?
- What factors affect the rate of a reaction?
- How does the surface area affect the rate of reaction?
- Explain the effect of concentration on the rate of reaction?
- What is the effect of a catalyst on the rate of a reaction?
- What is the collision theory in chemistry?
- How does the collision theory affect the rate of reaction?
Effect of temperature on rate of reaction experiment
Aim: To investigate the effect of temperature on the rate of reaction.
Problem statement: How does temperature affect the rate of reaction?
Hypothesis: An increase in temperature will increase the rate of reaction.
Variables:
(a) Manipulated variable : Temperature ot sodium thiosulphate solution
(b) Responding variable : Rate of reaction
(c) Controlled variables : Volume and concentration of sodium thiosulphate solution, volume and concentration of sulphuric acid, size of conical flask
Operational definition:
Rate of reaction is inversely proportional to the time taken for the mark ‘X’ to disappear from sight.

Materials: 0.2 mol dm–3 sodium thiosulphate solution, 1 mol dm–3 sulphuric acid, white paper with a mark ‘X’ at the centre.
Apparatus: 150 cm3 conical flasks, 50 cm3 measuring cylinder, 10 cm–3 measuring cylinder, digital stopwatch (electronically operated with an accuracy of 0.01 s), thermometer, Bunsen burner, tripod stand, wire gauze.
Procedure:

- 50 cm3 of 0.2 mol dm–3 sodium thiosulphate solution is measured using a measuring cylinder and poured into a conical flask.
- The temperature of this solution is measured using a thermometer.
- The conical flask is placed on top of a piece of white paper with a mark ‘X’ at the centre.
- 5 cm3 of 1 mol dm–3 sulphuric acid is measured using a 10 cm3 measuring cylinder.
- The sulphuric acid is then poured quickly and carefully into the conical flask and a stopwatch is started immediately.
- The mixture in the conical flask is swirled a few times. The conical flask is then placed back on the white paper.
- The mark ‘X’ is viewed vertically from the top through the solution, as shown in Figure.
- The stopwatch is stopped immediately once the mark ‘X’ disappears from sight.
- The time f required for the mark ‘X’ to disappear from sight is recorded.
- Steps 1 to 9 are repeated using 50 cm3 of 0.2 mol dm–3 sodium thiosulphate solution at 35°C, 40°C, 45°C and 50°C respectively by heating the solution as shown in Figure before 5 cm3 of 1 mol dm–3 sulphuric acid is added. All other conditions remain unchanged.
- The results are recorded in a table.
Results:
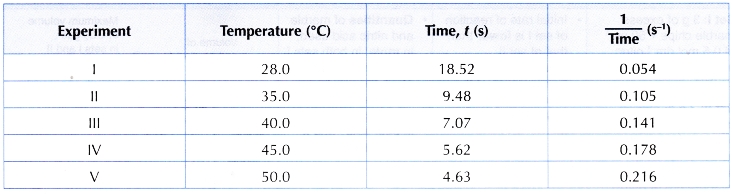
Interpreting data:
1. Based on the results, two graphs are plotted.
(a) Graph I: Graph of the temperature against time
(b) Graph II: Graph of the temperature against 1/time
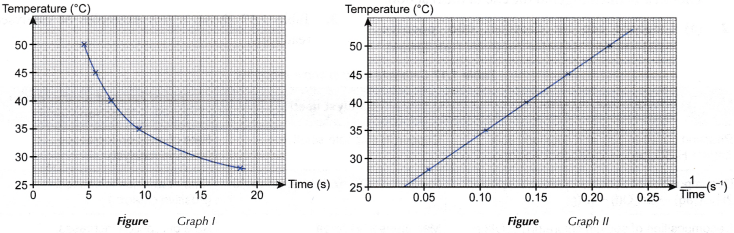
2. From graph I, it can be deduced that as the temperature increases, the time taken for the mark ‘X’ to disappear from sight becomes shorter.
3. From graph II, it can be deduced that the temperature increases linearly with 1/time.
Discussion:
- (a) From the graphs plotted, the following inference can be deduced:
“Temperature increases linearly with 1/time”
(b) But, rate of reaction ∝ 1/time.
(c) Hence, rate of reaction increases linearly with temperature. - In other words, as the temperature increases, the rate of reaction also increases.
- Usually, the rate of a reaction approximately doubles for every 10°C rise in temperature.
- The concentration and volume of both sodium thiosulphate solution and sulphuric acid are kept constant in each set of the experiment. Any change in rate of reaction is due to the difference in temperature.
Conclusion:
Rate of reaction increases when the temperature of the reaction increases. Hence, the hypothesis can be accepted.
