How does the size of particles affect the rate of reaction?
Effect of surface area on the rate of reaction:
- When the particle size of a fixed mass of a solid reactant becomes smaller, the total exposed surface area becomes larger, the rate of reaction increases.
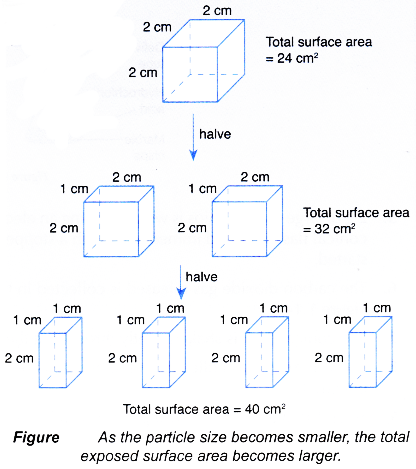
- For example, two sets of experiments are carried out using the reacting conditions below:
Set I: 1 g of zinc powder and 20 cm3 of 0.2 mol dm–3 hydrochloric acid at room conditions.
Set II: 1 g of granulated zinc and 20 cm3 of 0.2 mol dm–3 hydrochloric acid at room conditions. - The rate of reaction for set I is higher than that for set II.
- This is because 1 g of zinc powder used in set I has a larger total exposed surface area than 1 g of granulated zinc.
- For example, two sets of experiments are carried out using the reacting conditions below:
- When investigating experimentally the effect of particle size/surface area on the rate of a reaction,
- the experiment is carried out twice
- the particle size/surface area of a solid reactant used in the two experiments are varied
- all the other factors/conditions are kept constant in both the experiments.
- (a) The reaction between reactive metals and dilute acids to liberate hydrogen gas can also be used to study the effect of surface area on the rate of reaction.
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
(b) Rate of reaction of reactive metals such as magnesium, aluminium, zinc or iron with dilute acid is affected by the size of the metals.
(c) The smaller the size of a fixed mass of magnesium, aluminium, zinc or iron, the larger the total exposed surface area and the higher is the rate of reaction with dilute hydrochloric acid or dilute sulphuric acid. - An example of the effect of size of a solid reactant on the rate of reaction is shown in Table.
| Experiment | Initial rate of reaction | Maximum volume of hydrogen gas |
| Set I: 1 g of granulated iron + 50 cm3 of 0.2 mol dm–3 sulphuric acid Set II: 1 g of iron filings + 50 cm3 of 0.2 mol dm-3 sulphuric acid | Initial rate of reaction of set II is higher than that of set I because the total exposed surface area of 1 g of iron filings is larger than that of 1 g of granulated iron. | The quantities of iron and sulphuric acid used, in mole, in both sets I and II are the same. Thus, the maximum volume of hydrogen gas collected in both sets I and II are also the same. |
Graph:
People also ask
- What is the rate of the reaction?
- How do you calculate the reaction rate?
- What factors affect the rate of a reaction?
- Explain the effect of concentration on the rate of reaction?
- How does the temperature affect the rate of a chemical reaction?
- What is the effect of a catalyst on the rate of a reaction?
- What is the collision theory in chemistry?
- How does the collision theory affect the rate of reaction?
How surface area affects the rate of reaction experiment
Aim: To investigate the effect of total exposed surface area of a solid reactant on the rate of reaction.
Problem statement: How does the total exposed surface area of a solid reactant affect the rate of reaction?
Hypothesis: When the total exposed surface area of marble chips increases, the rate of reaction increases.
Variables:
(a) Manipulated variable : Total exposed surface area of marble chips
(b) Responding variable : Rate of reaction
(c) Controlled variables : Mass of marble, volume and concentration of hydrochloric acid, temperature
Operational definition:
- Smaller marble chips have a larger total exposed surface area than larger marble chips of the same mass.
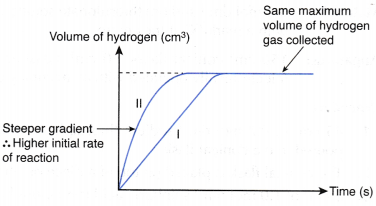
- For the graph of the volume of gas liberated against time, the curve with higher initial gradient indicates a higher initial rate of reaction.
Materials: 0.1 mol dm–3 hydrochloric acid, large marble chips, small crushed marble chips, water.
Apparatus: 100 cm3 measuring cylinder, 150 cm3 conical flask, stopper fitted with a delivery tube, basin, burette, electronic balance, stopwatch.
Procedure:
- A burette is filled with water until it is full. It is then inverted over water in a basin and clamped vertically using a retort stand.
- The water level in the burette is adjusted and the initial burette reading is recorded.
- 40 cm3 of 0.1 mol dm–3 hydrochloric acid is measured using a measuring cylinder and poured into a conical flask.
- The apparatus as shown in Figure is set up.
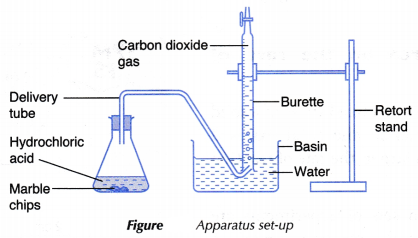
- 2 g of large marble chips is weighed using an electronic balance and added to the acid in the conical flask. The conical flask is closed immediately with a stopper fitted with a delivery tube. At the same time, a stopwatch is started.
- The carbon dioxide gas released is collected in the burette by downward displacement of water as shown in Figure.
- The conical flask is shaken gently throughout the whole experiment.
- The volume of gas collected in the burette is recorded at regular intervals of 30 seconds until no more gas is liberated.
- Steps 1 to 8 are repeated using 2 g of small crushed marble chips to replace 2 g of large marble chips. All the other conditions remain unchanged.
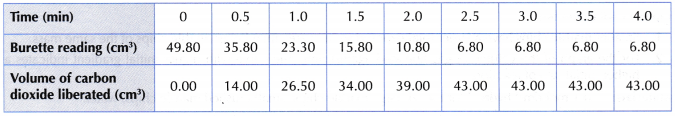
Results:
Using large marble chips:
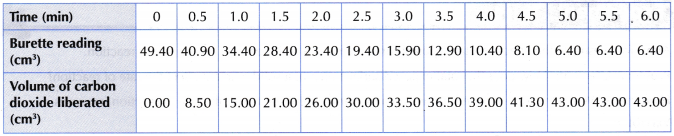
Using small crushed marble chips:
Interpreting data:
1. The graphs of volume of carbon dioxide gas liberated against time for both sets of the experiments are plotted on the same axes, as shown in Figure.
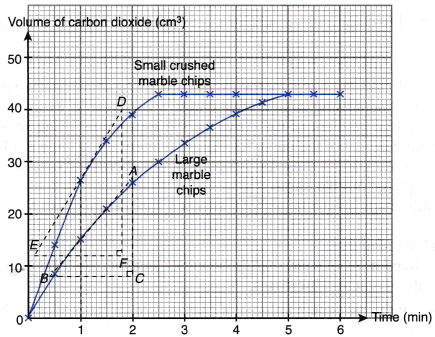
2. To calculate the instantaneous rate of reaction

(c) By comparison, the instantaneous rate of reaction at 1 minute of the experiment using small crushed marble chips is higher than that of the experiment using large marble chips.
3. To calculate the overall average rate of reaction

(c) By comparison, the overall average rate of reaction of the experiment using small crushed marble chips is higher than that of the experiment using large marble chips.
Discussion:
- The reaction between marble chips and hydrochloric acid releases carbon dioxide gas. The chemical equation for the reaction is:
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)- Based on the graphs plotted, the curve for the experiment using small crushed marble chips is steeper than the curve for the experiment using large marble chips.
- In other words, the initial gradient of the curve for the experiment using small crushed marble chips is higher than the initial gradient of the curve for the experiment using large marble chips.
- Thus, the inital rate of reaction for the experiment using small crushed marble chips is higher than that for the experiment using large marble chips.
- Small crushed marble chips have a larger total exposed surface area than the large marble chips with the same mass.
- Hence, it can be concluded that:
When the size of a fixed mass of solid marble becomes smaller, the total exposed surface area becomes larger, hence the rate of reaction increases.
- The above graph shows that the maximum volume of carbon dioxide gas collected in both experiments are the same, that is, 43.0 cm3.
- This is because the quantities of hydrochloric acid and marble used, in mole, are the same in both experiments.
- Theoretical maximum volume of carbon dioxide gas that should be collected can be calculated as follows:
Number of moles of hydrochloric acid used
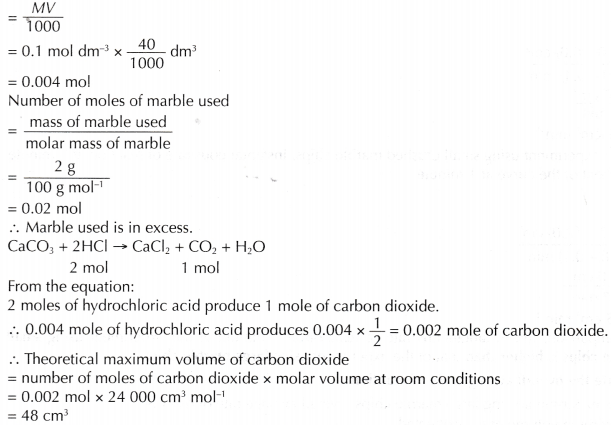
- The maximum volume of carbon dioxide gas collected in the experiment is less than the theoretical volume because a small volume of the carbon dioxide has dissolved in the water when it is collected in the burette.
- A method to overcome this difference is by saturating the water with carbon dioxide gas before collecting the gas in the burette.
Conclusion:
When the total exposed surface area of a solid reactant increases, the rate of reaction also increases. Hence, the hypothesis can be accepted.
