What is Sublimation in chemistry
Sublimation : The changing of a solid directly into vapours on heating, and of vapours into solid on cooling, is known as sublimation.
Sublimation can be represented as:
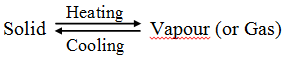 The solid substance which undergoes sublimation is said to ‘sublime’. the solid obtained by cooling the vapours of the solid is called a ‘sublimate’.
The solid substance which undergoes sublimation is said to ‘sublime’. the solid obtained by cooling the vapours of the solid is called a ‘sublimate’.
 Ex. When solid ammonium chloride is heated, it directly changes into ammonium chloride vapour. And when hot Ammonium chloride vapour is cooled, it directly changes into solid ammonium chloride. Ammonium chloride, Iodine, Camphor, Naphthalene and Anthracene.
Ex. When solid ammonium chloride is heated, it directly changes into ammonium chloride vapour. And when hot Ammonium chloride vapour is cooled, it directly changes into solid ammonium chloride. Ammonium chloride, Iodine, Camphor, Naphthalene and Anthracene.
Evaporation : The process of change of a liquid into vapour at any temperature below its boiling point is called evaporation.
Factors affecting evaporation :
- Temperature : Rate of evaporation increase with increase in temperature. This is because with the increase in temperature more number of particles get enough kinetic energy to go into the vapour state.
Ex. Drying of clothes take place rapidly in summer than in winter - Surface Area : The rate of evaporation increases on increasing the surface area of the liquid
Ex. If the same liquid is kept in a test tube and in a china dish, then the liquid kept in the china dish will evaporate more rapidly : Because more of its surface area is exposed to air. - Humidity : Humidity is the amount of water vapour present in air. Air around us cannot hold more than a definite quantity of water vapour at a given temperature. If the amount of water in air is already large i.e., humidity is more, the rate of evaporation decreases. Thus, the rate of evaporation increases with decrease in humidity in the atmosphere.
Ex. Drying of clothes on a humid day. - Wind speed : The rate of evaporation also increases with increase in speed of the wind. This is because with increase in speed of wind, the particles of water vapour move away with wind resulting decrease in the amount of vapour in the atmosphere.
Ex. Clothes dry faster on a windy day.
