What is an sp3 Hybridized Carbon atom
sp3 Hybridisation
In the excited carbon atom its one s-orbital (2s) and three p-orbitals (2px , 2py, 2pz) intermix and reshuffle into four identical orbitals known as sp3 orbitals. Thus, carbon atom undergoes sp3 hybridisation.
The four electrons enter into the new four identical hybrid orbitals known as sp3 hybrid orbitals one each as per Hund’s rule. (because they are made from one ‘s-orbital’ and three ‘p-orbitals’ they are called sp3 orbitals).
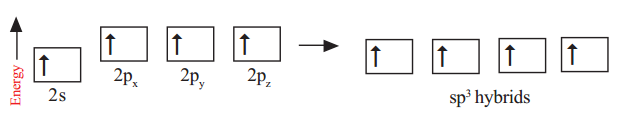
The hybridisation enables the carbon to have four identical sp3 hybrid orbitals and these have one electron each. Since carbon has four unpaired electrons, it is capable of forming bonds with four other atoms may be carbon or atoms of some other monovalent element. When carbon reacts with hydrogen, four hydrogen atoms allow their ‘s’ orbitals containing one electron each to overlap the four sp3 orbitals of carbon atom which are orieted at an angle of 109°28′. (Four orbitals of an atom in the outer shell orient along the four corners of a tetrahedron to have minimum repulsion between their electrons). The nucleus of the atom is at the centre of the tetrahedron. See figures below:
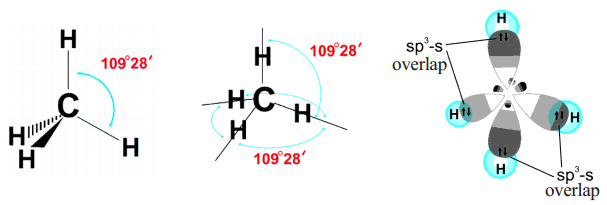
This leads to form four sp3 – s sigma bonds between carbon atom and four hydrogen atoms. All these bonds are of equal energy.
Read More:
- Binding of Carbon with other Elements
- Homologous Series of Hydrocarbons
- Allotropes of Carbon
- Catenation in Carbon
- Classification of Hydrocarbons
- Hybridization of the Carbon atoms in Acetylene
- Versatile Nature of Carbon
- What are the Characteristics of Compounds
- Chemical Properties of Carbon Compounds
- Nomenclature of Carbon Compounds
