How can we Separate a Mixture of a Solid and a Liquid using Evaporation
Separation of mixture of a solid and a liquid
All the mixtures containing a solid and a liquid are separated by one of the following processes:
Separation by filtration : The process of removing insoluble solids from a liquid by using a filter paper is known as filtration. Filtration is used separating insoluble substances from a liquid. The liquid passes through the filter paper and collected in the beaker kept below the funnel. The solid particles do not pass through the filter paper and remain behind on the filter paper. The solid substance left behind on the filter paper is called residue. The clear liquid obtained is called filtrate.
Ex. A mixture of chalk and water is separated by filtration.
Separation by centrifugation : We can separate the suspended particles of a substance in a liquid very rapidly by using the method of centrifugation. Centrifugation is done by using a machine called centrifuge. Centrifugation is a method for separating the suspended particles of a substance from a liquid in which the mixture is rotated at a high speed in a centrifuge.
In the method of centrifugation, the mixture of fine suspended particles in a liquid is taken in a test-tube. The test-tube is placed in a centrifuge machine and rotated rapidly for some time. As the mixture rotates round rapidly, a force acts on the heavier suspended particles in it and brings them down to the bottom of the test-tube. The clear liquid, being lighter, remains on top
Ex. We can separate the clay particles suspended in water very rapidly by the method of centrifugation. The suspension of clay particles in water is taken in a test tube and rotated very fast in a centrifuge machine. the clay particles settle down at the bottom of the test-tube and clear water remains at the top.
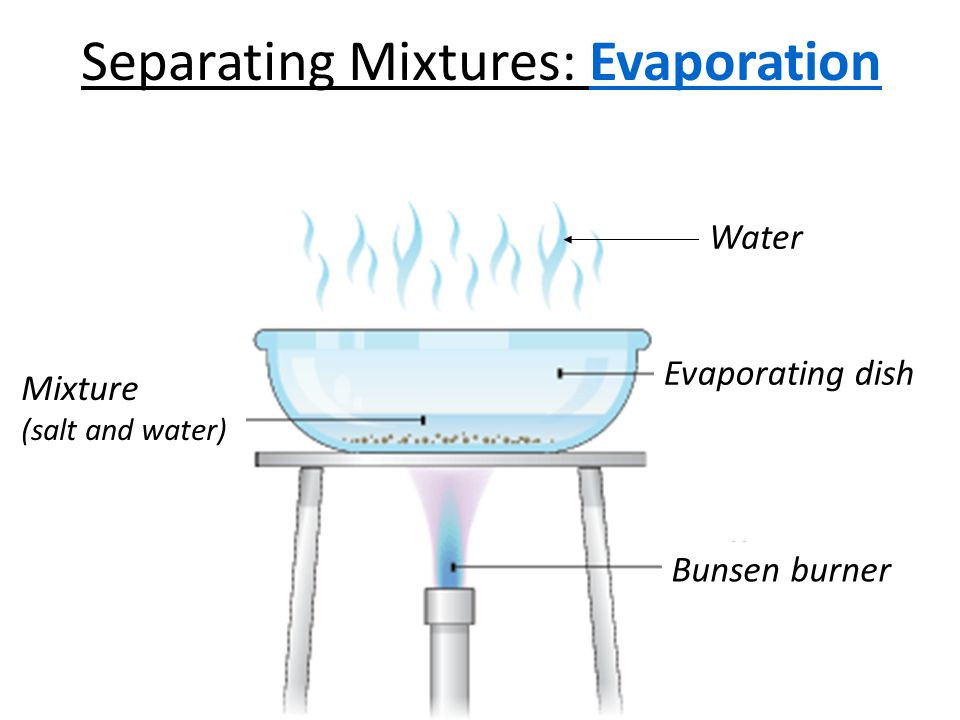
Separation by Evaporation : The changing of liquid into vapours is called evaporation. Evaporation is used to separate
a solid substance that has dissolved in water (or any other liquid). The dissolved substance is left as a solid residue when all the water
(or liquid) has evaporated. The use of process of evaporation for separating a mixture is based on the fact that liquids vapourise easily whereas solids do not vapourise easily. Though evaporation of a liquid can take place even at room temperature but it is very slow at room temperature. Evaporation can be made quicker by heating the solution.
If we have a mixture of common salt and water, then we cannot separate common salt from water by filtration or centrifugation. This is because common salt is completely dissolved in water and not insoluble in it. We can recover common salt from salt-water mixture (or salt solution) by the process of evaporation.
Ex. The common salt dissolved in water can be separated by the process of evaporation. The solution of common salt and water is taken in a china dish and heated gently by using a burner. The water present in salt solution will form water vapours and escape into atmosphere. When all the water present in the solution of common salt and water gets evaporated, then common salt is left behind in the china dish as a white solid.
 The process of evaporation is used on a large scale to obtain common salt from sea-water.
The process of evaporation is used on a large scale to obtain common salt from sea-water.
Purification by crystallisation : The process of cooling a hot, concentrated solution of a substance to obtain crystals is called crystallisation. The process of crystallisation is used for obtaining a pure solid substance from impure sample.
- The impure solid substance is dissolved in the minimum amount of water to form a solution.
- The solution is filtered to remove insoluble impurities.
- The clear solution is heated gently on a water bath till a concentrated solution or saturated solution is obtained (This can be tested by dipping a glass rod in hot solution from time to time. When small crystals form on the glass rod, the solution is saturated). Then stop heating.
- Allow the hot, saturated solution to cool slowly.
- Crystals of pure solid are formed. Impurities remain dissolved in solution.
- Separate the crystals of pure solid by filtration and dry.
Separation by chromatography :
Chromatography is a technique of separating two (or more) dissolved solids which are present in a solution in very small quantities. By using paper chromatography, we can separate two (or more) different substance present in the same solution. This separation is based on the fact that though two (or more) substances are soluble in the same solvent but their solubilities may be different. Some may be more soluble than the others.
Ex. Black ink is a mixture of several coloured substances which can be separated by paper chromatography.
Separation by distillation : Distillation is the process of heating a liquid to form vapour, and then cooling the vapour to get back liquid. Distillation can be represented as :
Liquid Vapour (or Gas)
The liquid obtained by condensing the vapour is called ‘distillate’. When the homogeneous mixture of solid and a liquid is heated in a closed distillation flask, the liquid, being volatile, forms vapour. the vapours of liquid are passed through a ‘condenser’ where they get cooled and condense to form pure liquid. This pure liquid is collected in a separate vessel. The solid, being non-volatile, remains behind in the distillation flask.
Ex. Salt-solution can be separated into salt and water by distillation.
