Selina ICSE Solutions for Class 9 Chemistry – Elements, Compounds and Mixtures
ICSE SolutionsSelina ICSE Solutions
Download Formulae Handbook For ICSE Class 9 and 10
Selina ICSE Solutions for Class 9 Chemistry Chapter 3 Elements, Compounds and Mixtures
Exercise 3(A)
Solution 1.
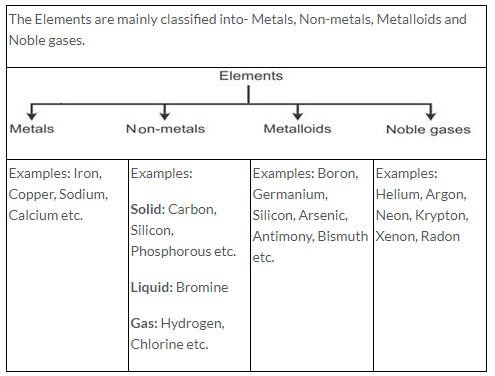
An element is a pure substance composed of only one kind of atom. Example: C, H, O, Na, Ca, N etc.
Characteristics of an element:
- An element is made up of only one kind of atoms.
- An element is pure and homogeneous substance.
- An element has fixed melting and boiling points.
- An atom is the smallest particle of an element which takes part in a chemical reaction.
- An element may chemically react with another elements or compounds.
- An element can occur in solid, liquid or gaseous state.
- The molecules are made up of one or more atoms of the same or different elements.
Solution 2.
Solution 3.
Two elements which show exception to the properties of:
Metals :-
- Mercury (Hg) is liquid at room temperature.
- Tungsten (W) is a poor conductor of electricity.
Non-metals:-
- Iodine is lustrous.
- Carbon is ductile.
Solution 4.
(a) Molecule: A molecule is the smallest particle of a pure substance (element or compound), and it has all the properties of that substance. It is composed of atoms. It is capable of existing in a free state.
Example: O2, H2, Cl2 are molecules.
(b) Atomicity: Atomicity is the number of atoms present in a molecule of an element.
(c) Compound: A compound is a pure substance composed of two or more elements combined chemically in a fixed proportion by mass. The properties of compounds are different from the properties of their constituent elements. Example: H2O, CO2 etc.
Solution 5.
| (a) A diatomic element | Nitrogen (N2) |
| (b) A tetratomic element | Phosphorus (P) |
| (c) Monoatornic element | Helium (He) |
| (d) Lustrous non-metal | Iodine |
| (e) Liquid non-metal | Bromine (Br2) |
| (f) A gas filled in electric bulbs | Argon (Ar) |
| (g) A liquid metal | Mercury (Hg) |
| (h) A non metal conductor of electricity | Graphite |
| (i) A metal non malleable and non ductile | Zinc (Zn) |
| (j) A lustrous non metal | Graphite |
Solution 6.
(i) Sodium chloride is obtained when sodium chemically combines with chlorine in ratio of 23:355 by weight.
(ii) When molten sodium chloride is subjected to electrolysis, the ratio by weight of sodium and chlorine librated at electrodes is 2:3.
Solution 7.
| Type | Substances | Reason |
| Element | Chlorine, Sulphur | They cannot be split up into any simpler substance. |
| Compound | Carbon dioxide | It can be produces by chemical analysis of two or more simpler substances with different properties. |
| Mixture | Honey, milk, sea water, gun powder, apple juice, brine, syrup and bronze | These are produced by mere mixing of two or more substances in any proportions by weight. |
Solution 8.
(a) This is because molecules have all the properites of that substance and is capable of existing in a free state, molecules are composed of atoms.
(b)
| Element | Compund |
| 1. It is a pure substance which cannot be converted into simpler substances by any physical or chemical means. | 1. It is a pure substance made up two or more elements combined chemically in a fixed ratio. |
| 2. It is made up of only one kind of atoms. | 2. It is made up of two or more different kinds of atoms. |
| 3. The molecules are made up of one or more atoms. | 3. The molecules are made up of two or more atoms. |
Solution 9.
It is true that the elements can form different compounds.
Example: Hydrogen and oxygen combine to give two different compounds, water (H2O) and hydrogen peroxide (H2O2) under different conditions.
Solution 10.
Characteristics of a compound
- A compound is made up of one or more atoms of the same or different elements.
- It has a homogeneous composition.
- In a compound the elements are present in a fixed ratio by mass.
- The properties of a compound are different from those of its Constituent elements.
Solution 11.
The properties of compounds are different from the properties of their constituent elements. Example: H2O, FeS, C12H22O11
- H2O: Water is a liquid, while constituent elements, Hydrogen and Oxygen are gases.
- FeS: Iron sulphide is a black substance, not attracted by a magnet and insoluble in carbon disulphide. While constituent elements, Iron is grey colored, attracted by a magnet. Sulphur is a yellow colored, soluble in carbon disulphide.
- C12H22O11: Sugar is a crystalline solid, sweet to taste and soluble in water. But, its constituent elements, Carbon, is black insoluble solid. Hydrogen and Oxygen are invisible and odorless gases.
Solution 12.
A compound is a pure substance composed of two or more elements combined chemically in fixed proportions by mass. The properties of a compound are different from the properties of their constituent elements.
- H2O: Water is a liquid, while constituent elements, Hydrogen and Oxygen are gases.
- FeS: Iron sulphide is a black substance, not attracted by a magnet and insoluble in carbon disulphide. While constituent elements, Iron is grey colored, attracted by a magnet. Sulphur is a yellow colored, soluble in carbon disulphide.
- C12H22O11: Sugar is a crystalline solid, sweet to taste and soluble in water. But, its constituent elements, Carbon, is black insoluble solid. Hydrogen and Oxygen are invisible and odorless gases.
Solution 13.
A mixture cannot be represented by a chemical formula because constituents present in a mixture are in any ratio and they are not chemically united.
Solution 14.
(a) Air
(b) Concrete
(c) Milk
Solution 15.
| Elements | Compounds | Mixtures |
| Mercury | Sugar, Distilled water, Alcohol, Nitre, Washing soda, Rust, Marble | Air, Milk, Wax, Sea-water, Paint, Brass, Bread, Soap, Tap water |
Solution 16.
On adding sulphuric acid to water we will get a Homogeneous Mixture (true solution).
This mixture will have different densities and boiling points depending upon the amounts of acid and water. The properties of acid and water will remain same even after mixing.
Solution 17.
Iron and sulphur when mixed, forms a mixture. It can be identified as follows:
- A grayish yellow mixture will be produced.
- All the individual properties of iron andsulphur will be shown separately in a mixture. Iron particles will be attracted by magnet. Sulphur will dissolve in carbon disulphide. Iron and sulphur when heated forms a compound.
It can be identified as follows:
- A grey dark solid will be produced.
- The compound formed is homogeneous.
- It is neither attracted by a magnet nor it is soluble in carbon disulphide.
Solution 18.
| Mixture | Compound |
| 1. It is obtained by the physical combination of either elements, or compounds, or both. | 1. It is obtained by the chemical combination of more than one element. |
| 2. The composition of elements present in a mixture is not fixed. | 2. The composition of elements present in a compound is fixed. |
| 3. It shows the properties of all its constituent elements. | 3. The properties of a compound are different from those of its elements. |
| 4. Its constituents can be separated using physical methods. | 4. Its constituents can be separated by using only chemical and electrochemical methods. |
| 5. The mixtures can be homogeneous or heterogeneous. | 5. A compound is always homogeneous in nature. |
Solution 19.
| No. | Types of mixture | Example | Nature |
| 1. | Two solids | Bronze (Zn, Cu, Sn) | Homogeneous |
| 2. | A solid in liquid | Sugar in water, Salt in water, Iodine in alcohol Sugar in oil | Homogeneous Heterogeneous |
| 3. | Liquids | Oil in water, Kerosene in water, Acetone + water | Heterogeneous Homogeneous |
| 4. | Liquid and gas | Moisture in air | Homogeneous |
Solution 20.
| Homogeneous mixtures | Heterogeneous mixture |
| 1. A mixture is said to be homogeneous if its constituents are uniformly distributed and are not physically distinct. | 1. A mixture is said to be heterogeneous if its constituents are not uniformly distributed and are physically distinct. |
| 2. They have the same composition and properties throughout their mass. | 2. They have the different composition and properties in different parts of their mass. |
| 3. For example: sugar solution, salt in water etc. | 3. For example: Mixture of sand in water, Mixture of oil in water |
Solution 21.
(a)
- The properties of ammonia are different from those of its components i.e nitrogen and hydrogen.
- When ammonia is formed from nitrogen and hydrogen, energy is given out.
- In ammonia, N and H are present in fixed ratio of 14 : 3 by mass.
(b) Air is considered as mixture not a compound because:
- The composition of air is different at different places and different altitudes.
- The constituents of air can be easily separated by physical means.
- The properties of air vary according to the properties of oxygen and nitrogen.
(c) Tap water does not have a fixed melting point. It may have various dissolved as well as undissolved impurities. Also it does not have a fixed composition.
Solution 22.
A pure substance is a homogeneous material with a definite, invariable chemical composition, and definite, invariable physical and chemical properties.
Sugar is a pure substance as all sample of sugar consists of same chemical composition. i.e. carbon, hydrogen and oxygen, C12H22O11.
Solution 23.
If dilute hydrochloric acid is added to the compound formed of iron and sulphur, Hydrogen sulphide gas is produced. (With a rotten egg smell).
Solution 24.
Solution 25.
When a mixture of powdered iron and sulphur is heated in a test tube, the powder starts melting and a pungent smell of a gas is given out. A dark grey solid is produced and this is called iron sulphide.
Solution 26.
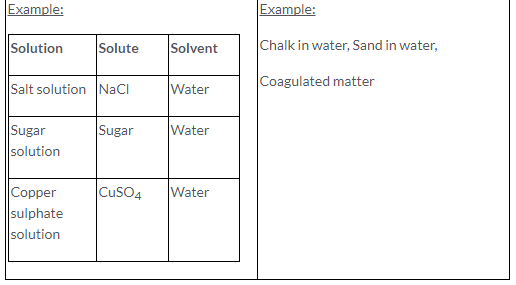
Solute: A substance which gets dissolved in a solvent is called as solute.
Solvent: A substance in which solute gets dissolved in it is called as solvent.
Solution: A homogeneous mixture of two or more substances which are chemically non reacting, whose composition can be varied within certain limits is called a solution.
Solution = Solute + Solvent
Solution 27.
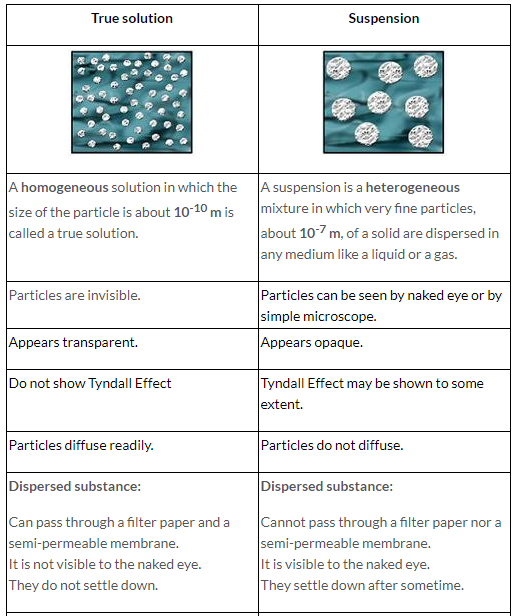
A homogeneous solution in which the size of the particle is about 10-10 m is called a true solution.
Properties of true solution:
- It is a homogeneous mixture.
- The solute particles are very small, about 10-10 m.
- It is clear and transparent.
- It does not scatter light:
- The components cannot be separated by filtration or by any physical means.
- The solute particles in a solution do not settle down.
- The particles are invisible.
- The particles diffuse rapidly
Solution 28.
Properties of suspension are:
- Suspension is a heterogeneous mixture.
- The solute particles in a suspension have a size of greater than 10-7 m.
- It is opaque in nature.
- The particles are visible to naked eye or under a simple microscope.
- The particles do not pass even through ordinary filter paper.
- The particles of a suspension do not diffuse.
- The particles in a suspension settle down on standing.
- It scatters light to some extent.
Solution 29.
A homogeneous looking heterogeneous mixture in which particles having a size between 10-10 m and 10-7 m dispersed in a continuous medium is called as colloid.
| Sr. no. | Dispersion medium | Dispersed phase | Common name of the system | Examples |
| 1. | Solid | Gas | Solid foam | Pumice stone, foam rubber etc. |
| 2. | Liquid | Gas | Foam or froth | Soap lather, shaving cream foam, lemonade froth, etc. |
| 3. | Liquid | Liquid | Emulsion | Milk, emulsified oils, medicines etc. |
| 4. | Solid | Liquid | Gel | Cheese, Butter, Jams, Jellies, Boot polish etc. |
| 5. | Gas | Liquid | Aerosol of liquids | Fog, mist, cloud, Liquid sprays etc. |
| 6. | Solid | Solid | Solid sol | Glasses, Gems, Pigmented plastics etc. |
| 7. | Liquid | Solid | Sol | Sulphur sol, Gold sol, Ferric hydroxide sol, Starch solution, Muddy water etc. |
| 8. | Gas | Solid | Aerosol of solids | Smoke, dust etc. |
Solution 30.
The movement of colloidal particles towards a particular electrode under the influence of an electric field is called electrophoresis.
It is shown by only those solutions, the particles of which carry a charge, such as colloid, particles. Thus this phenomenon of electrophoresis can be used to find the nature of charge carried by colloidal particles in a colloidal system.
For example, If the colloidal particles carry positive charge, they move. Towards negatively charge electrode (cathode) when subjected to an electric field. If they carry negative charge, they move towards positively charged electrode (Anode).
Solution 31.
| Sr no. | Property | True solution | Suspension | Colloids |
| 1 | Particle size | Less than 10-10 m | Greater than 10-7m | Between 10-10 to 10-7 m |
| 2 | Filtrability | Pass easily through ordinary filter paper as well as animal membranes. | Do not pass even through ordinary filter filter paper animal membranes. | Pass easily through ordinary paper but not through animal membranes. |
| 3 | Visibility of particles | Invisible | Visible to naked eye or under simple microscope. | Visible under Ultra microscope. |
Solution 32.
A suspension is a heterogeneous mixture in which very fine particles, about 10-7 m, of a solid are dispersed in any medium like a liquid or a gas.
Dispersed substance:
- Cannot pass through a filter paper nor a semi-permeable membrane.
- It is visible to the naked eye.
- They settle down after sometime.
Examples:
Chalk in water, Sand in water, Coagulated matter.
Solution 33.
Tyndall effect can be defined as, the scattering of beam of light by colloidal particles present in a colloidal solution.
Tyndall effect can be observed when a fine beam of light passes through a small hole in the dark room. This happens due to the scattering of light by the particles of dust or smoke present in the air.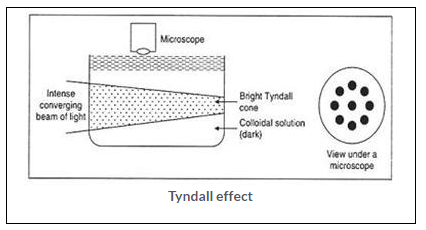
Experiment- In two Separate jars, take (FeSO4) Ferrous sulphate solution (green colour) in A and milk solution diluted with water in B.
Focus a torch on these jars in dark place. Torch light will not be visible in Ferrous sulphate solution and will not show Tyndall Effect as FeSO4 solution is actually true solution whereas light will be visible in B jar containing milk solution which is colloidal or Emulsion.
Examples of Tyndall effect from our daily life :-
- Tyndall Effect can be observed when sun light passes through the Canopy of a dense forest. In the forest, mist contains tiny droplets of water, which act as colloidal particles dispersed in air.
- When light enters a room through d small slit, dust particles scatter the light.
- Tyndall effect is observed when colloidal solution is distinguished from a true solution.
Solution 34.
The effect is called as the Tyndall effect.
Tyndall effect is shown by colloidal solution.
Properties of colloidal solution:
- The particles of colloidal solution do not settle under gravity. They can be made to settle down by centrifugation.
- It is a heterogeneous mixture.
Solution 35.
(a) Liquid in water – e.g. Alcohol solution, ammonia solution.
(b) Non-aqueous solution – e.g. solution of iodine in alcohol- This is called Tincture iodine. (c) Solid in non-aqueous solvent – e.g. solution of stearicacid in ethanol.
Solution 36.
Common names of colloids:
- Solid -gas is Solid foam
- Solid -Liquid is Gel
- Liquid – Solid is Sol
Solution 37.
| Solution | Mixture | Compound |
| 1. The solute is not present in any fixed proportion but its composition is uniform. | The composition is not uniform. The component may be present in any proportion. | The constituents of a compound are combined in a definite proportion. |
| 2. The solute can be recovered by evaporating the solvent, i.e. by physical means. | The components can be separated by ordinary physical means. | The components can be separated by chemical means only |
Solution 38.
The distributed substance in the solution is called as dispersed phase.
The medium in which distributed substance is dispersed is referred to as dispersion medium.
Solution 39.
Blood – colloidal solution Sugar solution – true solution Salt solution – true solution
Starch solution – colloidal solution Ink – colloidal solution
Solution 40.
Starch solution, milk, soap solution, blue vitriol will scatter light as these are colloidal solutions.
Exercise 3(B)
Solution 1.
The methods used to separate solid-liquid mixture are:
- Evaporation
- Distillation
- Filtration
- Sedimentation and decantation
- Centrifugation /churning
- Chromatography
Solution 2.
(a) Sand and water are separated by Filtration. Since sand is insoluble in water and forms Heterogeneous mixture. Water dissolves sodium chloride. The solution is than filtered with the help of a filter paper. Sand gets collected as a residue on the filter paper. And the dissolved sodium chloride in water can later be evaporated.
(b) Salt from an aqueous salt solution – Salt can be separated from aqueous salt solution by the process of evaporation. For this the aqueous salt solution (i.e. salt in water) is taken in a beaker and heated over flame. The liquid i.e. water will get evaporated leaving behind salt.
(c) Pure water from salt water- Pure water can be separated from salt water by the process of distillation. Distillation is the process of converting a liquid into vapour by heating and the subsequent condensation of the vapour back into a liquid. Water evaporates and recondenses in pure form and it is collected in a receiver. The salt residue remains in the distillation flask.
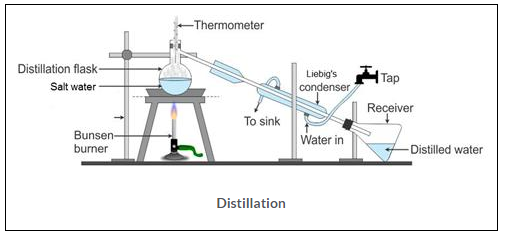
(d) Tea leaves from prepared tea – Tea leaves can be separated from prepared tea by the process of filtration. For this purpose a sieve or mesh made of steel or nylon is taken through which prepared tea is passed, thereby leading to retaining of tea leaves on the mesh.
(e) Cream from milk – Cream can be separated from milk by the process of centrifugation. The mixture is taken in centrifuge tubes and placed in holders. The holders are then rotated rapidly. After sometime, the rotation is stopped and the centrifuge tubes are taken out. It is observed that separation of mixture takes place on the bases of density. i.e. the more denser components settles at the bottom and the other becomes the supernatant which can be separated. In case of mixture of cream and milk. Cream comes in the upper layer and is recovered from milk.
(f) Sugar from sugar solution – It is separated by crystallisation. Heat the solution to obtain a saturated solution. Filter the solution while hot and allowed to cool. Crystals of sugar will be formed which are collected.
(g) Dye from black ink – Dye from black ink is separated by the process of chromatography. In this the constituents of a mixture are separated by their absorption over an appropriate absorbing material.
Solution 3.
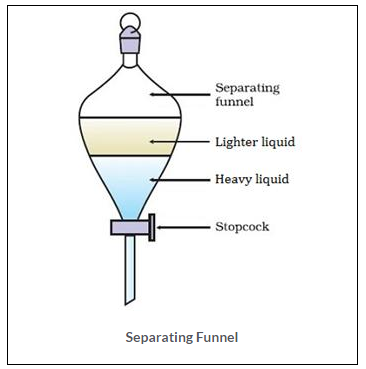
(a) Carbon – titrachloride and water form immiscible mixture and can be separated by Separating Funnel. Since, they are immiscible liquids and forms two distinct layers.
Carbon tetrachloride is heavier liquid with the density of 1.59 g/cm3. Water is lighter with the density of 1g/cm3. Thus, the denser liquid carbon tetra chloride forms a lower level in the funnel which can be separated out first. The remaining water can be collected in the separate beaker.
(b) Lead chloride and silver chloride can be separated by solvent extraction. For this the given mixture is dissolved in water. Silver chloride dissolves in water but lead chloride does not dissolve. The solution is filtered through a filter medium, lead chloride will be retained on the filter medium and the filtrate containing water and silver chloride is collected. Then the filtrate is evaporated to get silver chloride.
Solution 4.
The method of separation depends on both the type of mixture and the physical properties of its constituents. These are :
(i) The physical state of the constituents.
(ii) The differences in the physical properties of the constituents such as :
(a) boiling point, (b) melting point, (c) density, (d) magnetic properties, (e) ability to sublime, (f) volatility, (g) solubility in various solvents .
Solution 5.
It is the process of dissolving one of the components in a particular liquid when one of the components is soluble in water or in some other solvents and the other component is not.
For example, a mixture of iron filings and sulphur is separated by using carb disulphide as solvent A mixture of sodium chloride and chalk can also be separated by using water as the solvent for sodium chloride.
Solution 6.
(a) Mercury, silver
(c) Magnesium, sodium carbonate (NO2CO3)
(b) Calcium, lead
(d) Sodium
Solution 7.
(a) Sublimation: Sublimation is the change of state of matter from solid to gaseous state without passing through the liquid state.
For example, To separate the mixture of ammonium chloride and sodium chloride, sublimation is used. On heating ammonium chloride will change into vapour which will condense into a solid in the neck of the inverted funnel which can be scrapped off. Sodium chloride remains in the evaporating dish.
(b)
| Evaporation | Sublimation |
| 1. Evaporation is change of state matter from solid state to gaseous state. 2. Evaporation takes place below its boiling point by supply of heat. | 1. Sublimation is the change of state of matter from solid state to gaseous state. 2. Sublimation takes place below its melting point by supply of heat. |
Solution 8.
(a) Distillation is the process of converting a liquid into vapour (by heating) and the subsequent condensation of the vapour back into a liquid.
(b) Fractional distillation is a process which involves distillation and collection of fractions or different liquids boiling at different temperatures.
(c) Centrifugation is the method of separating solids from a liquid, where the mixture is homogeneous.
Solution 9.
(a) Two miscible liquids can be separated by-
- Distillation or by
- Fractional Distillation depending on difference in their boiling points.
(b) Yes, mixture of chloroform (B. P. 61C) and carbon-tetrachloride (B. P. 77C) can be separated by Fractional Distillation as difference in their B. P. is less than 30C. Chloroform has a lower boiling point 61oC, so, it distils out first. It is collected in a receiver, leaving behind carbon tetrachloride (having boiling point 77oC).
Modification: A fractionating G-Column is fitted over distilling flask.
(c) Homogeneous Mixtures of solid and liquid are separated by Centrifugation.
Uses of Centrifugation
- For separating cream from milk and butter from curd in dairies.
- In washing machines to squeeze out water from the wet clothes.
Solution 10.
(a) Dissolve the mixture of carbon and sulphur in carbon disulphide. Sulphur gets dissolve in carbon disulphide. The undissolved solid (carbon) is removed by filtration.
The filtrate is evaporated to dryness in order to recover the soluble solid i.e. sulphur powder.
(b) (i) Separation of potassium chloride: Prepare the suspension of mixture in water. The potassium chloride dissolves, but neither carbon nor sulphur, Filter the suspension and collect clear filtrate of potassium chloride in a separate beaker. Evaporate the clear filtrate on low heat. The water evaporates leaving behind white potassium chloride.
(ii) Separation of sulphur: Dissolve the residue in carbon disulphide. Sulphur dissolves in carbon disulphide leaving behind carbon. Filter the suspension and collect clear filtrate. Evaporate the filtrate in shade. The carbon disulphide evaporates leaving behind sulphur.
(iii) Removal and purification of carbon: Wash the residue of carbon on filter paper with carbon disulphide, so as to remove any sulphur. Dry the residue in shade. Carbon disulphide evaporates leaving behind carbon.
Solution 11.
(a) Chromatography
(b) Sedimentation
(c) Evaporation
(d) Fractional distillation
Solution 12.
By Sublimation method
Ammonium chloride and potassium chloride are placed in an evaporating dish and covered with an inverted funnel. On heating ammonium chloride will change into vapour which will condense into a solid in the neck of the inverted funnel which can be scrapped off. Sodium chloride remains in the evaporating dish.
Solution 13.
When caustic soda is added to an aqueous solution of copper sulphate, a blue precipitate of Cu(OH)2 , is obtained. Cu(OH)2 will be separated from mixture by filtration.
Solution 14.
Three commercial materials obtained from fractional distillation of petroleum are as follows:
- Natural gases
- Kerosene oil
- Lubricating oil
Use of Natural gases:
- It is used as fuel.
Use of Kerosene oil:
- It is used as fuel in homes.
- It is used for lighting the lamps on roads and home.
Use of lubricating oil:
- It is used to lubricate different parts of machines and vechic1es.
- It is used to prepare black polish etc.
Solution 15.
(a) By Sublimation: Heat the impure iodine in a china dish over a low flame. When violet fumes of iodine starts coming out. Place a cold inverted funnel over the china dish. The violet fumes condense on the cooler sides of funnel to form tiny crystals of pure iodine.
(b) By Magnetic Separation: Roll a strong horse shoe magnet on the mixture. The iron filings cling to the magnet, leaving behind c
Solution 16.
(a) Alcohol from a mixture of alcohol and water can be separated through fractional distillation. Alcohol liberates first because it has a lower boiling point than water.
(b) The components which are soluble in water but their solubilities are different.
For example, (i) Sodium nitrate and sodium chloride. (ii) Potassium chlorate and potassium chloride.
Solution 17.
(a) Nitre and common salt:
Take a beaker and prepare a saturated solution of the mixture in boiling water. On cooling, nitre will crystallize out while common salt will remain in solution. The process is repeated two or three times to separate the components completely.
(b) Ammonia and hydrogen:
The mixture of gases is bubbled through a Woulfe’s bottle containing the solvent i.e. water in which Ammonia dissolves. The insoluble gas component i.e. hydrogen passes out through the delivery tube and is collected in gas jar. The soluble gas component is obtained from its aqueous solution by boiling the solution.
(c) Powdered Chalk and Sugar:
Dissolve given mixture in water. Sugar dissolves but chalk does not as it is insolube chalk. Filter the mixture. Chalk is residue and is dried in the folds of filter paper. Filtrate is crystallized to get crystals of sugar.
(d) Sand, table salt:
Dissolve mixture in water. Salt dissolves but sand do not. Filter the solution to collect sand on filter paper. Then the filtrate is evaporated to get salt.
Iron filings and naphthalene:
By magnetic separation. Iron fillings get cling to the magnet and are separated. Remaining will be naphthalene.
Solution 18.
(a) Ingredients of gun-powder are – (nitre,sulphurand charcoal):
Dissolve mixture in water. Nitre dissolves but not sulphur and charcoal. Filter the mixture and collect the clear filtrate of nitre. Heat filtrate to crystallisation point. On cooling crystals of nitre separate out. Filter the crystals and dry them.
Dissolve the sulphur and charcoal in Carbon disulphide. Sulphur dissolves but charcoal does not. Filter the solution. Allow the filtrate to evaporate in shade. Carbon disulphide evaporates leaving behind sulphur. Charcoal is dried on filter paper.
(b) Sulphur, Sand and common salt:
Dissolve mixture in water. Common salt dissolves but sulphur and sand are insoluble in water. Filter the solution. sulphur and sand will get collected on the filter paper. Evaporate the solution to get common salt Collect the residue (Sand and Sulphur powder)and dissolve it in carbon disulphide, CS2. Sulphur dissolves in carbon disulphide but sand is insoluble in carbon disulphide. Filter the solution and evaporate it to get sulphur. Sand is dried on filter paper.
(c) Carbon dioxide and Carbon Monoxide:
When mixture is passed through a long tube having a number of porous partitions, Carbon monoxide will diffuse more rapidly as compared to Carbon dioxide. Thus if there is sufficient partitions, in the end, Carbon dioxide comes out.
(d) Water and Sugar:
Take a beaker half filled with water and dissolve as much sugar as you can in it, with constant stirring. Now heat the solution and go on adding sugar till it stops dissolving. Filter solution while it is hot. Allow the solution to cool. The crystals of pure sugar settle down at the bottom of the beaker.
(e) Sand and Iodine:
The mixture is placed in a china dish and an inverted dry funnel is placed over it, with its stem closed with cotton wool. It is then gently heated at a low flame. Iodine sublimes on the cooler side of funnel in the form of fine powder crystals. The residue left behind in the china dish is sand.
Solution 19.
Remove Iron using a Magnetic Separation:
Dissolve mixture in water. Sodium Chloride dissolves but not sulphur and charcoal. Filter the solution and allow the filtrate to evaporate to get Sodium Chloride. Now dissolve Sulphur and Charcoal in carbon disulphide solution, CS2. Sulphur dissolves but not charcoal. Filter the solution. Allow the solution to evaporate to get sulphur. Dry, charcoal in folds of filter papers.
Solution 20.
(a) solid-solid mixture:
- Magnetic separation: Example- Iron + Sulphur
- Solvent extraction: Example- Carbon + Sulphur
- Sublimation: Example- NH4Cl + NaCl
(b) Solid-liquid mixture:
- Evaporation: Example- Salt water solution
- Distillation: Example- Impure water
- Filtration: Example- Chalk + Water
(c) liquid-liquid mixture:
- Separating funnel: Example- Water + Carbon tetra chloride
- Fractional distillation: Example- Benzene + Toluene
- Distillation: Example- Acetone + Water
Solution 21.
The mixture is separated by exploiting the following facts in the order given below:
- Saw dust by gravity
- Ammonium chloride is soluble in water.
- Iodine sublime on heating
- Sand is insoluble in water
Solution 22.
(a) Dissolve given mixture in hot water lead chloride dissolves but not lead sulphate.
Filter the mixture. Residue is lead sulphate. Dry it in the folds of filter paper.
(b) Dissolve given mixture in water, Na2CO3, dissolves but not ZnCO3. Filter it ZnCO3 is residue and is dried in the folds of filter paper.
(c) Separation of given mixture can be done by fractional distillation. Boiling point of Benzene is 80°C and is less as compared with toluene having boiling point 111°C. So, Benzene distills over first.
(d) PbCl2 from a mixture of PbCl2 and AgCl.
Dissolve given mixture in water. AgCl dissolves, but not PbCl2. Filter the solution.
Evaporate the filtrate to get PbCl2.
Solution 23.
(a) Solid to gaseous state.
(b) Iodine
Solution 24.
Dissolve given mixture in dilute nitric acid. Copper oxide dissolves, but not charcoal. Filter the mixture, residue is charcoal. Dry it in the folds of filter paper. Now heat the filtrate to get copper oxide.
Solution 25.
To separate nitrogen from the air:
- Air is passed through filters or an electronic precipitator to remove dust. (ii) Then air is repeatedly compressed by increasing the pressure.
- Air is then cooled by decreasing the temperature.
- Now air becomes liquefied.
- Liquid air containing carbon dioxide, oxygen, nitrogen and inert gases is subjected to fractional evaporation.
- Carbon dioxide separates out as solid ice at -78°C.
- Liquid containing has a lower boiling point of -196°C, so it distills out first. Oxygen with boiling point -183°C is left behind.
Solution 26.
The two main components (oxygen and nitrogen) are separated from the air as follows:
- Air is passed through filters or an electronic precipitator to remove dust.
- Then air is repeatedly compressed by increasing the pressure.
- Air is then cooled by decreasing the temperature.
- Now air becomes liquefied.
- Liquid air containing carbon dioxide, oxygen, nitrogen and inert gases is subjected to fractional evaporation.
- Carbon dioxide separates out as solid ice at -78°C.
Solution 27.
(a) Chromatogeaphy: The process of separation of different dissolved constituents or mixture by absorbing them over an appropriate adsorbent material is called chromatography.
(b) The principle on which this technique is based is the difference in the adsorption different substances on the surface of a solid medium.
(c) Chromatograms are the distinct coloured rings or zones which are formed due to separation of mixture containing coloured substances by the process of chromatography.
(d) Advantages of chromatography:
- It can be carried out by very small amount of material.
- The substances under investigation do not get washed in chromatographic separation.
Solution 28.
(a) Centrifugation is used in diagnostic laboratories for testing blood/urine samples. It is also used for separation of cream from milk and butter from curd in dairies.
(b) Chromatography is used for purification of number of industrial products.
Solution 29.
The methods employed to separate two liquids are:
- Distillation and fractional distillation
- By using separating funnel.
Solution 30.
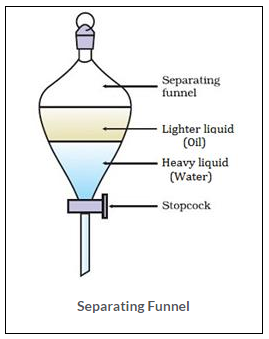
The apparatus used to separate oil and water is separating funnel. In this the two immiscible liquids are separated on the basis of their density differences. Thus, the heavier liquid will settle at bottom and the lighter one will form upper laye
Solution 31.
(a) Magnetic separation
(b) Solvent extraction
(c) Fractional crystallization
(d) Chromatography
(e) Boiling
(f) Separating funnel
(g) Distillation
(h) Evaporation
(i) Solvent extraction
Solution 32.
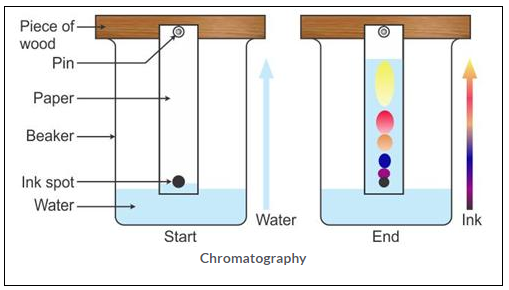
Take a rectangular sheet of filter paper approximately 10 em x 8em. Mark a horizontal line on the sheet about 2 cm from the lower edge and mark a cross (x) near the middle of the line. Prepare a mixture by mixing red and blue inks nearly-in equal proportions. With the help of dropper put about 2 to 3 drops of ink mixture on the cross (x) marked on the line. Allow it to dry. Fix the paper to a cork in a tall jar so that its lower end rests at the bottom of the jar. Pour a mixture of water and alcohol into the jar.
(1 : 1) so that about a 2 cm of filter paper dips in water-alcohol mixture. Leave it for about one hour. Solvent rises slowly by capillary action and reaches the cross marked on line and then goes on rising higher. Two coloured lines are seen at different heights. When the difference in heights is appreciable, remove the paper from jar and let it dry. In this way we can separate coloured constituents present in a mixture of ink.
Solution 33.
(a) sublimation
(b) filtration
(c) immiscible, separating funnel
(d) sublimation
(e) methylated sprit
