Selina Concise Chemistry Class 10 ICSE Solutions Electrolysis
APlusTopper.com provides step by step solutions for Selina Concise ICSE Solutions for Class 10 Chemistry Chapter 6 Electrolysis. You can download the Selina Concise Chemistry ICSE Solutions for Class 10 with Free PDF download option. Selina Publishers Concise Chemistry for Class 10 ICSE Solutions all questions are solved and explained by expert teachers as per ICSE board guidelines.
Download Formulae Handbook For ICSE Class 9 and 10
ICSE SolutionsSelina ICSE Solutions
Selina ICSE Solutions for Class 10 Chemistry Chapter 6 Electrolysis
Exercise Intext 1
Solution 1.
(a) Powdered sodium chloride (common salt) does not conduct an electric current, but it does so when dissolved in water or when melted.
(b) Molten lead bromide conducts electricity .It is called an electrolyte. It is composed of lead ions and bromide ions. The lead ions are positivelycharged and are called cations. The bromide ions are negatively charged and are called anions.
(c) Substances which conduct electricity in the solid state are generally metals.
(d) The electron releasing tendency of zinc is more than that of copper.
(e) A solution of HCl gas in water conducts electricity because it ionizes, but a solution of HCl gas in toluene does not conduct an electric current because it does not ionize in toluene.
Solution 2.
(a) Electrolysis: It is the process of decomposition of a chemical compound in aqueous solutions or in molten state accompanied by a chemical change using direct electric current.
(b) Non-electrolyte: It is a compound which neither in solution nor in the molten state allows an electric current to pass through it.
(c) Cation and anion: Atoms which carry positive charge are called cations.
Atoms which carry negative charge are called anions.
(d) Weak electrolyte: Electrolytes which allow small amount of electricity to flow through them and are partially dissociated in fused or aqueous solution are called weak electrolyte.
Solution 3.
(a) Difference between Modern explanation and Arrhenius explanation for the theory of electrolysis:
Arrhenius considered that water ionizes electrolytes but Modern theory explained that electrolytes are ionic even in solid state and their ions are held by strong electrostatic forces which make them immobile. Water renders these ions mobility by breaking the electrostatic forces.
(b) Difference between electrolytic dissociation and ionization :
| Ionisation | Dissociation |
| 1. Formation of positively or negatively charged ions from molecules which are not initially in the ionic state. | 1. Separation of ions which are already present in an ionic compound. |
| 2. Polar covalent compounds show ionization. e.g. HCl, H2CO3, NH4OH etc. | 1. Electrovalent compounds show dissociation. e.g. Potassium chloride , lead bromide, etc. |
(c) A cation and anion:
| Cation | Anion |
| 1. Are positively charged ions. | Are negatively charged ions. |
| 2. Migrate to cathode during electrolysis. | Migrate to anode during electrolysis. |
| 3. Gain electron from the cathode and get reduced to become a neutral atom. | Lose electrons to the anode and get oxidized to become a neutral atom. |
(d) Electrolytic dissociation and thermal dissociation:
Electrolytic dissociation is the dissociation of an electrovalent compound into ions in the fused state or in aqueous solution state.
Thermal dissociation: Reversible breakdown of a chemical compound into simpler substances by heating it. The splitting of ammonium chloride into ammonia and hydrogen chloride is an example. On cooling, they recombine to form the salt.
Solution 4.
(a) Sodium carbonate
(b) NH4OH
(c) An inert electrode: graphite and Active electrode: silver
(d) H+
(e) Electrode is cathode
(f) Graphite
Solution 5.
Electrolysis is a redox process. The reaction at the cathode involves reduction of cations as they gain of electrons while the reaction at anode involves oxidation of anions as they loss of electrons to become neutral.
Example: Dissociation of sodium chloride during electrolysis.
![]()
Cathode : Na+ + e– → Na (reduction)
Cl– – e– → Cl (oxidation)
Cl + Cl → Cl2
Overall reaction: 2NaCl → 2Na + Cl2
Exercise Intext 2
Solution 1.
(a) Glucose, Kerosene
(b) NaCl and NaOH
(c) CH3COOH and NH4OH
Solution 2.
(a) Cane sugar is a compound which does not have ions even in solution and contains only molecules. Hence, it does not conduct electricity. On the other hand, sodium chloride solution contains free mobile ions and allows electric current to pass through it. This makes it a good conductor of electricity.
(b) Hydrochloric acid is a strong electrolyte and dissociates completely in aqueous solution. The solution contains free mobile ions which allow electric current to pass through it. Hence, hydrochloric acid is a good conductor of electricity.
(c) Hydrogen is placed lower in the electrochemical series and sodium is placed at a higher position. This is because H+ ions are discharged more easily at the cathode than Na+ during electrolysis and gains electrons more easily.
Therefore, H+ ion is reduced at the cathode and not Na+ ion.
Solution 3.
(a) Zn occurs readily as ion whereas Cu occurs more readily as metal in nature.
(b) Copper is above silver in the electrochemical series and is thus more reactive than silver. So, copper displaces silver from silver nitrate. Hence, we cannot store AgNO3 solution in copper vessel.
Cu +AgNO3 → Cu(NO3)2 + 2Ag
(c) Copper is more active than Ag.
Solution 4.
(a) By treating its salt with a more reactive metal.
(b) By supplying two electrons to Cu+2
Cu+2 + 2e– → Cu
Solution 5.
In the aqueous state, the slightly negatively charged oxygen atoms of the polar water molecule exerts a pull on the positively charged sodium ions. A similar pull is exerted by the slightly charged hydrogen atoms of the water on the negatively charged chloride ions. Thus the ions become free in solution. These free ions conduct electricity.
In the molten state, the high temperatures required to melt the solid weakens the bond between the particles and the ions are set free.
Solution 6.
(a) Two anions are and OH–.
(b) OH– is discharged at anode and the main product of the discharge of OH– is O2
Reaction is :
OH– → OH + e–
4OH → 2H2O + O2
(c) The product formed at cathode is hydrogen. The reaction is :
H+ + e– → H
H + H → H2
(d) No change in colour is observed.
(e) Dilute sulphuric acid catalyse the dissociation of water molecules into ions, hence electrolysis of acidified water is considered as an example of catalysis.
Solution 7.
(a) Labelled diagram of electrolytic cell is:
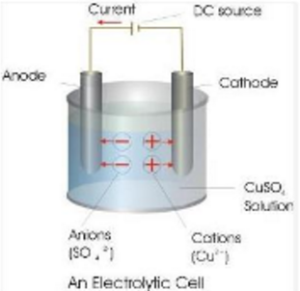
(b) The ions present in the cell are Cu2+, H+, SO42- , OH–.
(c) SO42- and OH– ions both migrate towards anode.
(d) Both Cu2+ and H+ ions migrate towards cathode.
(e) SO42- and H+ will not discharge at electrodes.
(f) Reaction at cathode:
Cu+2 +2e– → Cu
(g) Reaction at anode:
OH– – e– → OH
2OH + 2OH → 2H2O + O2
(h) Sulphate ions are the spectator ions because they do not change in the reaction.
Solution 8.
(a) Reaction at anode during the electrolysis ofvery dilute sulphuric acid:
OH– → OH + e–
4OH → 2H2O + O2
(b) Reaction at anode during the electrolysis of aqueous copper sulphate solution
4OH– → 4OH + 4e–
4OH → 2H2O + O2
(c) Reaction at anode during the electrolysis of sodium chloride solution
2Cl– → Cl2 + 2e–
(d) Reaction at anode during the electrolysis of fused lead bromide
Br– – e– → Br
Br + Br → Br2
(e) Reaction at anode during the electrolysis of magnesium chloride (molten)
2Cl– → Cl2 +2e–
(f) Concentrated HCl,
HCl in the pure liquid state is unionised and hence does not conduct electricity.
(g) Very dilute HCl,
Cl– – e– → Cl
Cl + Cl → Cl2
Solution 9.
(a) Electrolyte
(b) Nickel
(c) Cathode
(d) Anode
(e) Cations
Exercise 1
Solution 1.
(a) During electrolysis of lead bromide, there is loss of electrons at anode by bromine and gain of electrons at cathode by lead. Thus oxidation and reduction go side by side. Therefore, it is a redox reaction.
PbBr2 → Pb+2 + 2Br–
(b) The blue colour of copper ions fades due to decrease in Cu+2 ions and finally the solution becomes colourless as soon as Cu+2 ions are finished.
(c) Lead bromide dissociate into ions in the molten state whereas it does not dissociate in solid state. The ions become free when lead bromide is in molten state but in the solid state the ions are not free since they are packed tightly together due to electrostatic force between them. Therefore, lead bromide undergoes electrolytic dissociation in the molten state.
(d) Aluminium has great affinity towards oxygen, so it is not reduced by reducing agent. Therefore it is extracted from its oxide by electrolytic reduction.
(e) As per electrolytic reactions, 4H+1 are needed at cathode and 4OH– at the anode and two molecules of water are produced at the anode. Hence for every two molecules of water, two molecules of hydrogen and one molecule of oxygen are liberated at the cathode and anode respectively.
![]()
(f) This is because HNO3 is volatile.
(g) Ammonia is a covalent compound. Therefore, it is unionized in the gaseous state but in the aqueous solution it gives NH4OH which is a weak electrolyte and dissociates into ions.
(h) Graphite is unaffected by the bromine vapours.
(i) Silver nitrate is not used as electrolyte for electroplating with silver because the deposition of silver will be very fast and hence not very smooth and uniform.
(j) Carbon tetrachloride is a liquid and does not conduct electricity because it is a covalent compound and there are no free ions present and contain only molecules.
Solution 2.
(a) Strong electrolyte : Dilute hydrochloric acid, dilute sulphuric acid, ammonium chloride, sodium acetate
(b) Weak electrolyte: Acetic acid, ammonium hydroxide
(c) Non-electrolyte: Carbon tetrachloride
Solution 3.
(a) Molecules
(b) Will not
Solution 4.
Water is a non-conductor of electricity and consists entirely of molecules. It can be electrolytically decomposed by addition of traces of dilutesulphuric acid which dissociate as H+ and SO42- ions and help in dissociating water into H+ and OH–, water being a polar solvent.
Solution 5.
| Anode | Electrolyte | Cathode | |
| Silver plating of spoon | Plate of pure clean silver | Solution of potassiumargentocyanide | Article to be electroplated |
| Purification of copper | Impure copper | Solution of coppersulphate and dilutesulphuric acid | Thin strip of pure copper |
Solution 6.
Electricity, Chemical
Solution 7.
(b) CuSO4 is preferred as an electrolyte.
(c) The copper anode continuously dissolves as ions in solution and is replaced periodically. The electrolyte dissociates into Cu+2 ions which migrate towards the iron object taken as the cathode and are deposited as neutral copper atoms on the cathode.
Electrolyte: Aqueous solution of nickel sulphate
Dissociation: CuSO4 → Cu2+ + SO42-
H2O → H+ + OH–
Electrodes:
Cathode: Article to be electroplated
Anode: Block of pure copper
Electrode reactions:
Reaction at cathode: Cu2+ + 2e–→ Cu (deposited)
Reaction at anode: Cu – 2e–→ Cu2+
Solution 1 (2004).
(a) X → X2+ + 2e– , Y + 3e– → Y3-
(b) Y2 + 3X → X3Y2
(c) (i) It is used for the electroplating of metals.
(ii) It is also used in purification of metals.
(d) Cathode, Anode
Solution 1 (2005).
(a) Because Copper is an electronic conductor as it is a metal.
(b) In solid sodium chloride, Na+ and Cl – ions are not free due to strong electrostatic forces of attraction among them. The ions, therefore are unable to move to any large extent when electric field is affected. Hence no current.
Solution 1 (2006).
(a) (i) The name of electrode A is Platinum anode and that of electrode B is platinum or copper cathode.
(ii) Anode act as oxidizing electrode.
(b) AgNO3 solution will turn blue.
Solution 1 (2007).
(i) Molten ionic compound: Strong electrolytes
(ii) Carbon tetrachloride: Non-electrolyte
(iii) An aluminium wire: Metallic conductor
(iv) A solution containing solvent molecules, solute molecules and ions formed by dissociation of solute molecules: Weak electrolyte
(v) A sugar solution with sugar molecules and water molecules: Non- electrolyte
Solution 1 (2008).
(a) The reaction takes place at anode. This is an example of oxidation.
(b) Cu+2 will discharge easily at cathode.
Reaction at cathode:
Cu+2 +2e– → Cu
(c) Carbon tetrachloride is a non-electrolyte because it is a covalent compound. It does not ionize and hence do not conduct electricity.
Solution 2 (2004).
(a) Non-electrolyte contains molecules.
(b) Molecules of HX and H+ and X– ions.
(c) Loss
(d) The electrolyte used for the purpose must contain the ions of metal which is to be electroplated on the article.
(e) The reaction at the cathode involves reduction of cations as they gain electrons to become neutral atoms while that at anode involves oxidation of anions as they lose electrons to become neutral.
Example: Dissociation of sodium chloride during electrolysis.
NaCl → Na+ + Cl–
At cathode: Na+ + e– Na (Reduction)
At anode: Cl– – e– → Cl(oxidation)
Cl + Cl → Cl2
Overall reaction: 2NaCl → 2Na + Cl2
Solution 2 (2005).
Hydrogen gas is released at cathode when acidulated water is electrolyzed.
Solution 2 (2008).
During the electrolysis of molten lead bromide. Lead is deposited at cathode.
More Resources for Selina Concise Class 10 ICSE Solutions
- Concise Chemistry Class 10 ICSE Answers
- Concise Physics Class 10 ICSE Answers
- Concise Biology Class 10 ICSE Answers
- Selina ICSE Class 10 Maths Solutions
