What is saponification in soap making?
What is soap?
- Soaps are sodium or potassium salts of long-chain fatty acids
- The general formula of a soap can be written as RCOO–Na+ or RCOO–K+, where R is an alkyl group usually containing 12 or 18 carbon atoms. R can be saturated or unsaturated.
Soap Formula Sodium laurate CH3(CH2)10COO–Na+ Sodium palmitate CH3(CH2)14COO–Na+ Potassium stearate CH3(CH2)16COO–K+ Potassium oleate CH3(CH2)7CH=CH(CH2)7COO–K+
The history of soap manufacturing
- In the past, soap was made by mixing animal fats with alkaline wood ashes.
- Large-scale commercial soap making occurred in 1791 when a French chemist, Nicholas Leblanc patented a process for making soda ash or sodium carbonate from common salt. The process yielded large quantities of quality soda ash.
- Twenty years later, Belgian chemist Ernest Solvay developed a process to further reduce the cost of obtaining soda ash, thereby reducing the cost of soap manufacturing.
Soap preparation process
- Soaps can be made from animal fats and vegetable oils.
- The animal fats most commonly used are fats from cows and goats.
- The vegetable oils often used are palm oil, olive oil and coconut oil.
- Soaps are prepared by hydrolysing fats or oils under alkaline condition. The reaction is called saponification.
- The saponification process involves boiling fats or oils with concentrated sodium hydroxide solution or concentrated potassium hydroxide solution to produce glycerol and the salts of fatty acids which are the soaps.
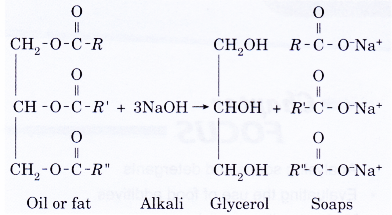
- The general equation for this reaction is:
where the three alkyl groups (R, R’ and R”) can be the same or different groups. - The fats or oils are hydrolysed first to form glycerol and fatty acids. The acids then react with an alkali to form the corresponding sodium or potassium salts.
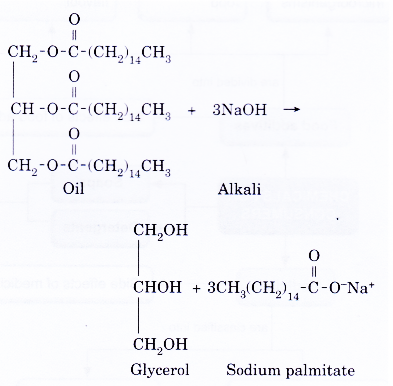
- The following equation shows how a soap, sodium palmitate, is prepared.
- When concentrated potassium hydroxide solution is used instead of concentrated sodium hydroxide solution, a potassium soap, potassium palmitate is formed.
- Potassium soaps are softer and milder than sodium soaps and are usually used for bathing.
People also ask
- How do you make detergent?
- Explain the cleansing action of soap and detergent
- How are soaps and detergents different?
- What are the Advantages of Synthetic Detergents over Soap?
- Types of food additives and their functions
- What are the different types of medicine?
- The Existence of Chemicals
Saponification process of making soap experiment
Aim: To prepare soap using the saponification process.
Materials: Palm oil, 5 mol dm-3 sodium hydroxide solution, sodium chloride powder, filter paper, distilled water.
Apparatus: 250 cm3 beaker, 50 cm3 measuring cylinder, spatula, glass rod, filter funnel, wire gauze, tripod stand,
Bunsen burner, test tube.
Safety Measures:
• Wear safety goggles.
• Concentrated sodium hydroxide solution is caustic. Avoid skin contact.
Procedure:
- 10 cm3 of palm oil is poured into a beaker.
- 50 cm3 of 5 mol dm-3 sodium hydroxide solution is added to the palm oil.
- The mixture is heated until it boils.
- The mixture is stirred with a glass rod.
- The mixture is allowed to boil for 10 minutes.
- The beaker is removed from the heat. 50 cm3 of distilled water and three spatulaful of sodium chloride are added to the mixture.
- The mixture is boiled for another 5 minutes.
- The mixture is allowed to cool.
- The soap is filtered out. The soap is washed with a little distilled water.
- The soap is pressed between a few pieces of filter paper to dry it.
- The soap is felt with the fingers. A small amount of the soap is placed in a test tube. Water is added into the
test tube. The mixture is then shaken. - The observations are recorded:
Observations:
The soap feels slippery. When the mixture Of the soap and water is shaken, lather is formed.
Discussion:
- When palm oil is heated with sodium hydroxide solution, a soap is formed. The word equation for the saponification process involved in this activity is:
Palm oil + concentrated sodium hydroxide solution → soap + glycerol - The soap formed can be precipitated by adding sodium chloride. This is because sodium chloride lowers the solubility of soap in water.
- The glycerol and excess sodium hydroxide solution are removed by rinsing the soap formed with water.
- Soaps have the following properties:
(a) Soaps feel slippery.
(b) Soaps form lather when they are shaken with water.
Conclusion:
Soaps can be prepared by heating palm Oil with concentrated sodium hydroxide solution.
