Relationship between pH values and molarity of acids and alkalis
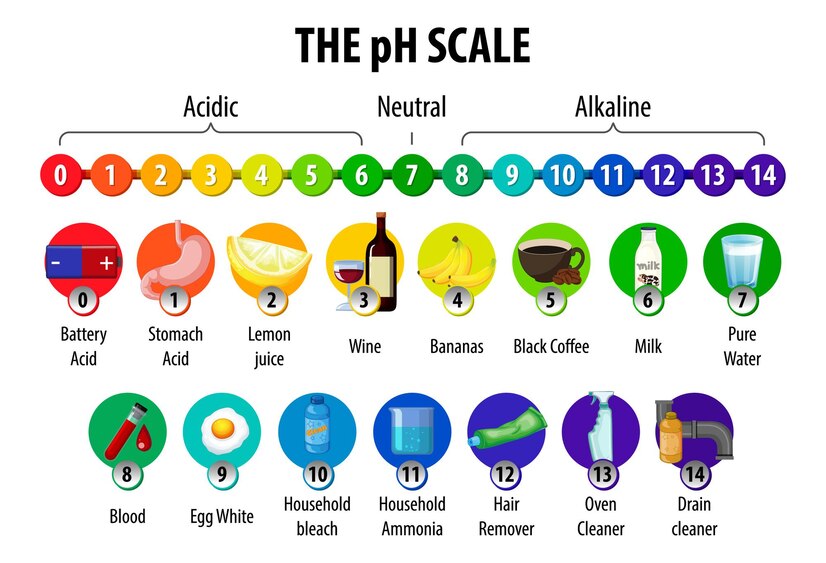
The relationship between pH values and concentration of hydrogen ions is given below:
- Concentration of hydrogen ions increases → pH value decreases
- In an acidic solution, the concentration of hydrogen ions depends on the concentration or molarity of the acidic solution. An acid with a higher molarity will have a higher concentration of hydrogen ions and hence a lower pH value.
Higher molarity of acid → lower pH value - Figure shows the pH values of acidic solutions of different molarity.
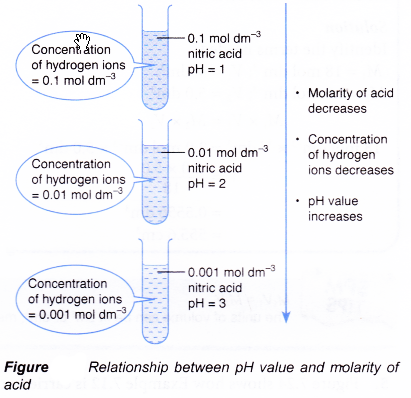
- The relationship between pH values and concentration of hydroxide ions is given below:
Concentration of hydroxide ions increases → pH value increases - In an alkaline solution, the concentration of hydroxide ions depends on the concentration or molarity of the alkaline solution.
- By increasing the molarity of an alkaline solution, concentration of hydroxide ions increases and its pH value increases.
Higher molarity of alkali → higher pH value - Figure shows how the pH values vary for different molarity of sodium hydroxide solutions.
The pH value of an acid or an alkali depends on:
- the strength (degree of ionisation) of acid/alkali
(i) stronger acid (higher degree of ionisation) → lower pH
(ii) stronger alkali (higher degree of ionisation) → higher pH - the concentration (molarity) of acid/alkali
(i) higher concentration (molarity) of acid → lower pH
(ii) higher concentraton (molarity) of alkali → higher pH - the basicity of acid/alkali
(i) higher basicity of acid → lower pH
(ii) higher basicity of alkali → higher pH
People also ask
- Concept of the pH Scale
- Role of pH in everyday life
- What is the pH of a salt solution
- What is the definition of an acid and a base?
- What is the definition of an acid in chemistry?
- What is the definition of a base in chemistry?
- Classification of Acids
- Preparation of Acids
- What are the chemical properties of an acid?
- General Properties of Acids
- Uses of Acids
- Preparation of Bases
- General Properties of Bases
- What determines a Strong Base and a Weak Base
- What are the uses of Bases
- How can we measure the strength of acids and alkalis?
- How to calculate concentration of acids and alkalis?
- How do you prepare a standard solution?
- What is meant by a neutralization reaction?
- How does titration determine concentration?
Relationship between pH values and molarity experiment
Aim: To investigate whether:
(a) an increase in the molarity of an acid will decrease its pH value and
(b) an increase in the molarity of an alkali will increase its pH value
Problem statement: (a) Does an increase in the molarity of an acid cause its pH value to decrease?
(b) Does an increase in the molarity of an alkali cause its pH value to increase?
Hypothesis: Increasing the molarity of an acid will decrease its pH value, whereas increasing the molarity of an alkali will increase its pH value.
Variables:
(a) Manipulated variable: Molarity of acid or alkali
(b) Responding variable: pH values
(c) Controlled variable: Types of acid or alkali
Materials: Hydrochloric acid with concentrations of 1.0 mol dm-3, 0.1 mol dm-3, 0.01 mol dm-3, 0.001 mol dm-3, and 0.0001 mol dm-3; sodium hydroxide solution with concentrations of 1.0 mol dm-3, 0.1 mol dm-3, 0.01 mol dm-3, 0.001 mol dm-3 and 0.0001 mol dm-3.
Apparatus: Boiling tubes, test tube rack and pH meter.
Procedure:
- Five boiling tubes are arranged in a test tube rack and labelled 1, 2, 3, 4 and 5.
- Each boiling tube is filled with about 10 cm3 of acid as follows.
- Boiling tube 1 – 1.0 mol dm-3 hydrochloric acid
- Boiling tube 2 – 0.1 mol dm-3 hydrochloric acid
- Boiling tube 3 – 0.01 mol dm-3 hydrochloric acid
- Boiling tube 4 – 0.001 mol dm-3 hydrochloric acid
- Boiling tube 5 – 0.0001 mol dm-3 hydrochloric acid
- A clean, dry pH meter is dipped info each acid to measure and record its pH value.
- The boiling tubes are cleaned and steps 1 to 3 are repeated using sodium hydroxide solution to replace the hydrochloric acid.
- The readings are recorded in Table.
Results:
| Boiling tube number | Hydrochloric acid | Sodium hydroxide solution | ||
| Molarity | pH | Molarity | pH | |
| 1 | 1.0 | 0 | 1.0 | 14 |
| 2 | 0.1 | 1 | 0.1 | 13 |
| 3 | 0.01 | 2 | 0.01 | 12 |
| 4 | 0.001 | 3 | 0.001 | 11 |
| 5 | 0.0001 | 4 | 0.0001 | 10 |
Discussion:
- Molarity measures the number of moles of acid or alkali in 1 dm3 of solution.
- Based on Table increasing the molarity of an acid decreases its pH value.
Reason:
Hydrochloric acid is a strong acid. When the molarity of hydrochloric acid increases,- the number of moles of hydrochloric acid dissolved in 1 dm3 of solution increases
- more hydrochloric acid molecules ionise to hydrogen ions
- the concentration of hydrogen ions also increases
- the pH value decreases
Hence, increasing the molarity of an acid will decrease its pH value.
- Based on Table, increasing the molarity of an alkali increases its pH value.
Reason:
Sodium hydroxide solution is a strong alkali. When the molarity of sodium hydroxide solution increases,- the number of moles of sodium hydroxide dissolved in 1 dm3 of solution increases
- more sodium hydroxide dissociates to produce hydroxide ions
- the concentration of hydroxide ions also increases
- the pH value increases
Hence, increasing the molarity of an alkali will increase its pH value.
Conclusion:
Increasing the molarity of an acid will decrease its pH value. Increasing the molarity of an alkali will increase its pH value. The hypothesis can be accepted.
