What is the Relationship between a Mole and Avogadro’s number
Avogadro’s number
Atoms and molecules are so small in size that they cannot be counted individually. The chemists use the unit mole for counting atoms, molecules or ions. It is represented by n.
A mole represents 6.022 × 1023 particles.
Example: 1 mole of atoms = 6.022 × 1023 atoms.
1 mole of molecules = 6.022 × 1023 molecules The number of particles present in 1 mole of any substance is fixed i.e. 6.022 × 1023.
This number is called Avogadro constant or Avogadro number. it is represented by No.
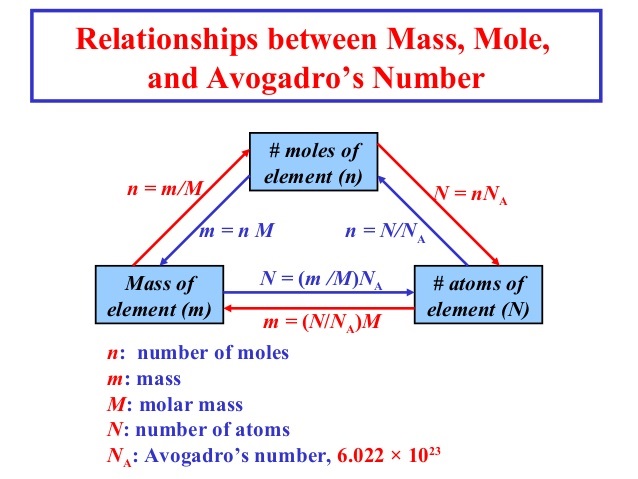
1 mole of atoms = 6.022 × 1023 atoms = Gram atomic mass or Molar mass of element
Number of moles = \(\frac { Mass\quad of\quad element }{ Molar\quad mass } \)
n = \(\frac { m }{ M } \)
Number of moles = \(\frac { Given\quad number\quad of\quad atoms }{ Avagadro\quad number } \)
n = \(\frac { N }{ { N }_{ 0 } } \)
No. of moles = n
Given mass = m
Molar mass = M
Given number of particles = N
Avogadro number of particles = N0
These relations can be interchanged as
Mass of element, m = n × M
or No. of particles of element, N = n × N0
Similarly,
1 Mole of molecules = 6.022 × 1023 molecules
= Gram molecular mass of Molar mass
Number of moles = \(\frac { Mass\quad of\quad substance }{ Molar\quad mass } \)
n = \(\frac { m }{ M } \)
Number of moles
= \(\frac { Given\quad number\quad of\quad molecules }{ Avagadro\quad number } \)
n = \(\frac { N }{ { N }_{ 0 } } \)
or m = n × M and N = n × N0
Relationship between mole, number of particles and mass and interconversion of one into the other.
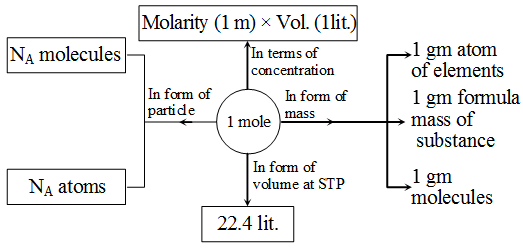
Molarity (M) : Moles of solute is one litre of solution is known as molarity .
M = \(\frac { Number\quad of\quad moles\quad of\quad solute }{ Volume\quad of\quad solution\quad in\quad litre } \)
Example 1: An ornament of silver contains 20 g of silver. Calculate the moles of silver present (atomic mass of silver = 180 u)
Solution: Moles of silver,
n = \(\frac { m }{ M } \)
Mass of silver, m = 20 g,
Molar mass of silver,
M = 108 g
∴ n = \(\frac { 20 }{ 108 } \) = 0.185 mol.
Example 2: How many moles of CO2 are present in 51.2 g of it ?
Solution: Molecular mass of CO2 = 12 + 2 + 16 = 44 u
Molar mass of CO2 (M) = 44 g
Mass of CO2 (m) = 51.2 g
Moles of CO2,
n = \(\frac { m }{ M } =\frac { 51.2 }{ 44 } \) = 1.16 mol.
Example 3: Calculate the mass of
(i) 0.5 moles of N2 gas
(ii) 0.5 moles of N atoms
Solution: (i) 0.5 moles of N2 gas
Mass = Molar mass × Number of moles
m = M × n
M = 28 g, n = 0.5
∴ m = 28 × 0.5 = 14 g
(ii) Mass = Molar mass × Number of moles
m = M × n
n = 0.5 mole, M = 14 g
m = 14 × 0.5 = 7 g
Mass percentage of an element from molecular formula :
The molecular formula of a compound may be defined as the formula which specifies the number of atoms of various elements in the molecule of the compound.
Example: The molecular formula of glucose is C6H12O6. This indicates that a molecule of glucose contains six atoms of carbon, twelve atoms of hydrogen and six atoms of oxygen.
The mass percentage of each element is then calculated by the following formula : Mass percentage of element X
= \(\frac { Mass\quad of\quad X\quad in\quad one\quad mole }{ Gram\quad molecular\quad mass } \times 100\)
Example 1: Calculate the percentage composition (by mass) of formaldehyde (CH2O).
Solution: Molecular mass of formaldehyde,
CH2O = 12 × 1 + 1 × 2 + 16 × 1 = 30
Mass of one mole of formaldehyde = 30 g
1 Mole of CH2O contains 1 mole (12 g) of carbon. 2 moles of hydrogen (2 g) and 1 mole of oxygen (16 g)
Percentage of carbon = \(\frac { 12g }{ 30g }\times 100\) = 40.0%
Percentage of hydrogen = \(\frac { 2g }{ 30g }\times 100\) = 6.7%
Percentage of oxygen = \(\frac { 16g }{ 30g }\times 100\) = 53.3%
Empirical formula
The empirical formula of a compound may be defined as the formula which gives the simplest whole number ratio of atoms of the various elements present in the molecule of the compound.
Example: The empirical formula of the compound glucose (C6H12O6), is CH2O which shows that C, H, and O are present in the simplest ratio of 1 : 2 : 1.
Rules for writing the empirical formula
The empirical formula is determined by the following steps :
- Divide the percentage of each elements by its atomic mass. This gives the relative number of moles of various elements present in the compound.
- Divide the quotients obtained in the above step by the smallest of them so as to get a simple ratio of moles of various elements.
- Multiply the figures, so obtained by a suitable integer, if necessary, in order to obtain whole number ratio.
- Finally write down the symbols of the various elements side by side and put the above numbers as the subscripts to the lower right hand corner of each symbol. This will represent the empirical formula of the compound.
Example: A substance, on analysis, gave the following composition : Na = 43.4%, C = 11.3%, O = 45.3%. Calculate its empirical formula
[Atomic masses = Na = 23, C = 12, O = 16]
Solution:
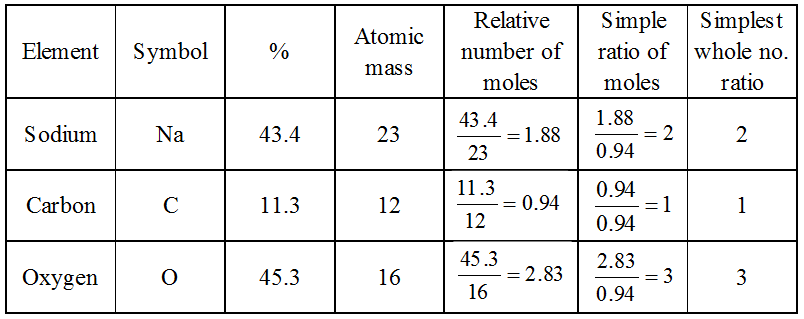
Therefore, the empirical formula is Na2CO3
Determination molecular formula :
Molecular formula = Empirical formula × n
n = \(\frac { Molecular\quad formula }{ Empirical\quad formula } \)
Example 1: What is the simplest formula of the compound which has the following percentage composition : Carbon 80%, Hydrogen 20%, If the molecular mass is 30, calculate its molecular formula.
Solution: Calculation of empirical formula :
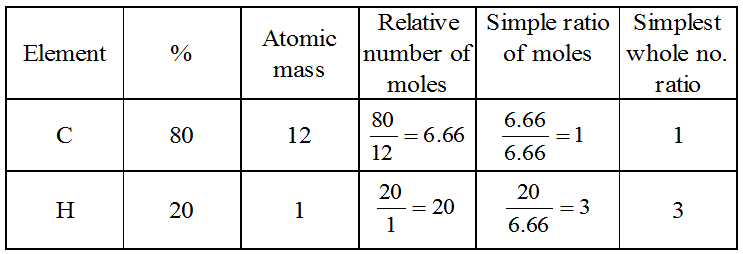
∴ Empirical formula is CH3.
Calculation of molecular formula :
Empirical formula mass = 12 × 1 + 1 × 3 = 15
n = \(\frac { Molecular\quad mass }{ Empirical\quad formula\quad mass } =\frac { 30 }{ 15 } \) = 2
Molecular formula
= Empirical formula × 2 = CH3 × 2 = C2H6.
Example 2: On heating a sample of CaCO3, volume of CO2 evolved at NTP is 112 cc. Calculate
(i) Weight of CO2 produced
(ii) Weight of CaCO3 taken
(iii) Weight of CaO remaining
Solution: (i) Mole of CO2 produced \(\frac { 112 }{ 22400 } =\frac { 1 }{ 200 } \) mole
mass of CO2 = \(\frac { 1 }{ 200 } \times 44\) = 0.22 gm
(ii) CaCO3 → CaO + CO2(1/200 mole)
mole of CaCO3 = \(\frac { 1 }{ 200 } \) mole
∴ mass of CaCO3 = \(\frac { 1 }{ 200 } \times 100\) = 0.5 gm
(iii) mole of CaO produced = \(\frac { 1 }{ 200 } \) mole
mass of CaO = \(\frac { 1 }{ 200 } \times 56\) = 0.28 gm
* Interesting by we can apply
Conversation of mass or wt. of CaO
= wt. of CaCO3 taken – wt. of CO2 produced
= 0.5 – 0.22 = 0.28 gm
Example 3: If all iron present in 1.6 gm Fe2O3 is converted in form of FeSO4. (NH4)2SO4.6H2O after series of reaction. Calculate mass of product obtained.
Solution: If all iron will be converted then no. of mole atoms of Fe in reactant & product will be same.
∴ Mole of Fe2O3 = \(\frac { 1.6 }{ 160 } =\frac { 1 }{ 100 } \)
mole atoms of Fe = 2 × \(\frac { 1 }{ 100 } =\frac { 1 }{ 50 } \)
mole of FeSO4. (NH4)2SO4.6H2O will be same as mole atoms of Fe because one atom of Fe is present in one molecule.
∴ Mole of FeSO4.(NH4)2.SO4.6H2O = \(\frac { 1 }{ 50 } \)
∴ Mass = \(\frac { 1 }{ 50 } \) × Molecule wt.
= \(\frac { 1 }{ 50 } \times 342\) = 7.84 gm.
Example 4: Calculate mass of hydrazine N2H4 obtained when 1.12 litre of N2 taken at NTP reacts with H2 according to N2 + 2H2 → N2H4.
Solution: Moles of N2 taken = \(\frac { 1.12 }{ 22.4 } =\frac { 1 }{ 20 } \)
N2 + 2H2 → N2H4
(1/20 mole) (1/20 mole)
mass of N2H4 = \(\frac { 1 }{ 20 } \times 32\) = 1.6 gm
Example 5: Calculate mass of Na2SO4 obtained when
100 ml of 0.2 M H2SO4 is completely neutralised by NaOH.
Solution: Mole of H2SO4 taken =
Molarity × Vol. (lit) = \(\frac { 100 }{ 1000 } \times 0.2\) = 0.02
H2SO4 + 2NaOH → Na2SO4 + 2H2O
(0.02 mole) (0.02 mole)
Mole of Na2SO4 obtained = 0.02
mass of Na2SO4 = 0.02 × 142 = 2.84 gm
