Redox reaction in the displacement of metals from its salt solution
- Generally, metals are good electron donors and therefore are good reducing agents. However, different metals have different strength as reducing agents.
- The strength of metals as reducing agents can be compared by using the electrochemical series.
- The electrochemical series lists metals according to their electropositivity, that is, according to their ability to lose electrons to form positive ions.
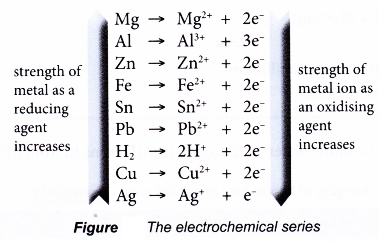
- The higher the position of a metal in the electrochemical series, the more electropositive the metal is, the easier it is for the metal to lose its electrons. Thus, the better reducing agent the metal is.
- On the other hand, the ability of a metal ion to accept electrons increases down the series. Thus, the strength of a metal ion as an oxidising agent increases down the electrochemical series.
- In a displacement of metal, a more electropositive metal will displace a less electropositive metal from its salt solution.
(a) The more electropositive metal acts as the reducing agent. It loses electrons and undergoes oxidation to form positive ions.
(b) The ions of the less electropositive metal act as an oxidising agent by accepting the electrons. While doing so, the ions are reduced to metallic atoms.
(c) In short, there is an electron transfer from the more electropositive metal to the ions of the less electropositive metal.
Oxidation and reduction in the displacement of metals experiment
Aim: To investigate oxidation and reduction in the displacement of metals.
Materials: Zinc strip, copper strip, 0.5 mol dm-3 copper(II) sulphate solution, 0.1 mol dm-3 silver nitrate solution, sandpaper.
Apparatus: Test tubes, test tube rack.
Procedure:
- 2 cm3 of 0.5 mol dm-3 copper(II) sulphate solution and 2 cm3 of 0.1 mol dm-3 silver nitrate solution are poured into two separate test tubes.
- A strip of zinc and a strip of copper are cleaned with sandpaper. The strips are then dropped into the test tubes as shown in Figure.
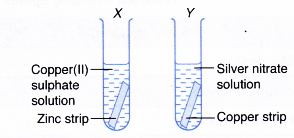
- Any change in colour and whether any metal is deposited are observed.
Observations:
| Test tube | Observations |
| X |
|
| Y |
|
Discussion:
- In test tube X, zinc displaces copper from its salt solution.
(a) Zinc is more electropositive than copper. Thus, zinc acts as the reducing agent, losing electrons to form zinc ions. By doing so, zinc is oxidised. This explains why the zinc strip dissolves.
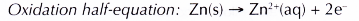
(b) The electrons are accepted by copper(II) ions in the solution. Thus, copper(II) ions act as the oxidising agent and are reduced to metallic copper. The brown solid deposited in test tube X is copper metal.
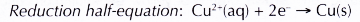
(c) The decreasing amount of copper(II) ions in the solution causes the solution to slowly change colour from blue to colourless.
(d) The redox reaction that occurs can be represented by the following equation.
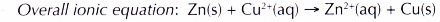
- In test tube Y, copper displaces silver from its salt solution.
(a) Copper is more electropositive than silver. So, copper acts as the reducing agent, losing electrons to form copper(II) ions. In other words, copper is oxidised. This explains why the copper strip dissolves.
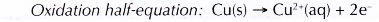
(b) The increasing amount of copper(II) ions in the solution causes the solution to slowly change colour from colourless to blue.
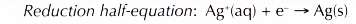
(c) The electrons are accepted by silver ions in the solution. Thus, silver ions act as the oxidising agent and are reduced to silvery grey silver.
(d) The redox reaction that occurs can be represented by the following equation.
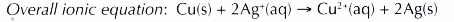
Conclusion:
A more electropositive metal can displace a less electropositive metal from its salt solution whereby the more electropositive metal acts as the reducing agent and the ions of the less electropositive metal act as the oxidising agent.
People also ask
- What is a redox reaction?
- Changing of iron(II) ions to iron(III) ions and vice versa
- Displacement of Halogen From Halide Solution
- Redox Reactions by Transfer of Electrons at a Distance
- Rusting as a Redox Reaction
- The Reactivity Series of Metals Towards Oxygen
- Application of the reactivity series of metals in the extraction of metals
- Electrolytic and Chemical Cells
- Oxidation and Reduction in Electrolytic Cells
- Oxidation and Reduction in Chemical Cells
- How does a voltaic cell work?
