Realising the Importance of Proper Management of Radioactive Substances
Realising the Importance of Proper Management of Radioactive Substances:
- Radiation may be defined as energy travelling through space. It can be classified as:
(a) Non-ionising radiation
(b) Ionising radiation
- Examples of non-ionising radiation are radio waves, microwaves, infrared rays, visible light and ultraviolet rays.
- Ionising radiation consists of:
(a) High energy particles such as protons, neutrons, α-particles and β-particles
(b) High frequency electromagnetic waves such as X-rays and γ-rays.
People also ask
- Radioactivity: Types of Radioactive Emissions
- How do you detect radioactivity?
- What are the different types of radioactive decay?
- What are the Isotopes, Isobars and Isotones of an Element
- What is the Radioactive Isotope?
- What is the half life of a radioactive element?
- What is nucleus of an atom?
- What is Nuclear Energy?
- What is nuclear fission and how does it occur?
- How is energy released in a nuclear fusion reaction?
- What happens in a nuclear chain reaction?
- How does a nuclear power plant works?
- What are the Sources of Energy?
Negative Effects of Radioactive Material
The Negative Effects of Radioactive Substances:
- Ionising radiation is extremely dangerous to living organisms.
- Ionising radiation transfers energy to the atoms and molecules in the living tissue of our bodies causing them to become ionised or excited.
- These ionisations and excitations can:
(a) Produce free radicals
(b) Break chemical bonds
(c) Produce new chemical bonds and cross-linkages between macromolecules
(d) Damage molecules that regulate vital cell processes (such as DNA, RNA and proteins)
- The cell can repair certain levels of cell damage. At low doses of radiation, cellular damage is rapidly repaired.
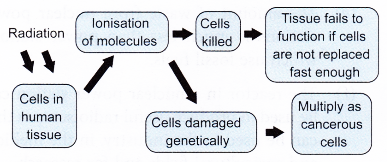
- At higher levels, cell death occurs. If the cells cannot be replaced quickly enough, tissues will fail to function.
- The ionisation effect of radiation can also cause genetic damage to the molecules of the cells. This may lead to the formation of cancerous cells and tumour development.
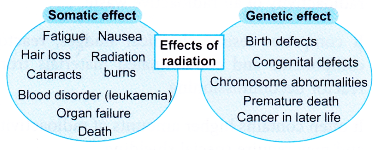
- Figure summarises the negative effects of radiation.
- If a radioactive substance gets inside the body, the most harmful effects come from a-particles because they have the highest ionisation power.
- If the radioactive source is outside the body, the greatest danger is from gamma sources because y-rays have the highest penetrating power. Alpha particles cannot penetrate clothing and is highly unlikely to reach living cells in the body.
- Our bodies are always exposed to natural background radiation. Figure shows the various sources of background radiation.
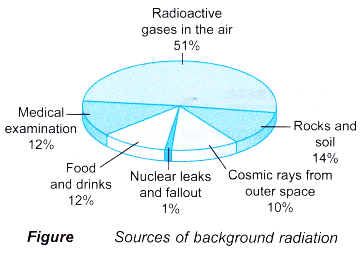
- The level of background radiation is very low and is not harmful to humans.
- In biological systems, radiation damage is divided into two categories: somatic damage and genetic damage.
- Somatic damage appears in the person exposed to radiation. The seriousness of the damage depends on the dose of radiation received.
- Genetic damage affects the reproductive cells and can lead to defective offspring in the future generations of the exposed person. Figure shows some of the negative effects of radiation.
Safety Precautions when Handling Radioactive Materials
Safety Precautions in the Handling of Radioactive Substances:
- People who handle radioactive substances must observe the correct procedures and safety precautions strictly. This is to ensure the safety of themselves, other people and the environment.
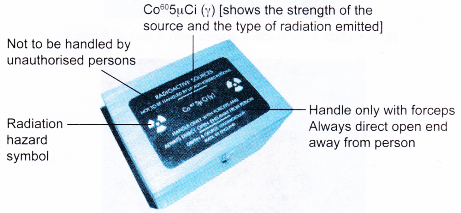
- Advice and instructions marked on radioactive sources, equipment and work manuals must be read and understood before beginning any work with radioactive substances. Figure shows the label on the box containing a radioactive source.
- Gloves must be worn when an unsealed source is used to avoid direct contact with the substance.
- Long-sleeved laboratory coats, long pants and shoes with socks should be worn.
- Eating, drinking, applying cosmetics or storing food in radiation laboratories is strictly prohibited.
- All work surfaces and storage areas (such as table tops and floors) should be covered with absorbent material to contain radioactive material contamination.
- When using radioactive liquids, plastic or stainless steel trays should be utilised to contain the liquid if a spill occurs.
- Radioactive material, especially liquids, should be kept in unbreakable containers.
- Hands and forearms should be washed thoroughly after working with radioactive substances.
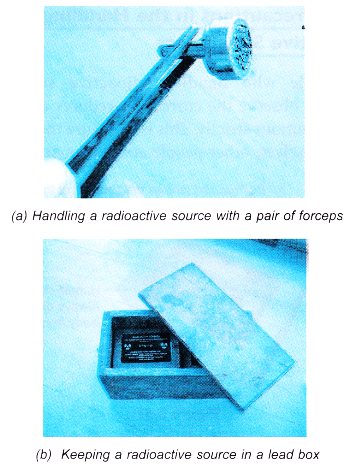
- Radioactive sources for educational use should be handled with forceps and kept in lead boxes as shown in Figure.
- Stronger radioactive sources should be handled with robotic control systems behind steel, concrete, lead or thick lead glass panels.
- Radiation badges worn by people who work in radiation laboratories or places where radioactive substances are used are regularly developed to monitor the amount of radiation the person has been exposed to over a period of time. If it exceeds the radiation dose limit that a normal healthy person can take, he or she will be reassigned to tasks where risks of further exposure is low.
Radioactive Waste Management
- Radioisotopes are widely used in many fields such as in scientific research, in the industry and in medicine. Used radioisotopes remain radioactive and have to be disposed off properly.
- In nuclear power stations, spent fuel rods are very hot and highly radioactive. They contain radioisotopes with very long half-lives which span up to thousands of years.
- Radioactive waste must be managed and stored carefully according to strict safety procedures.
- Radioactive waste is classified into three categories so that they can be managed efficiently:
(a) Low-level waste
(b) Intermediate-level waste - Three general principles are used in the management of radioactive wastes:
(a) Concentrate-and-contain
The waste is compacted to a smaller volume and stored in an isolated place.
(b) Dilute-and-disperse
The waste is diluted to safe levels of concentration and discharged to the environment
(c) Delay-and-decay
The waste is stored is a safe place and left to decay until it reaches a safe level of radioactivity. This could take many years.
Low-level Waste
- It is the most common form of waste. It comprises 90% of the volume but only 1% of the radioactivity of all radioactive waste.
- It comes mainly from hospitals, laboratories the industry as well as from the nuclear fuel cycle. Examples are paper, rags, gloves, tools, clothing and filters.
- It contains small amounts of mostly short-lived radioactivity.
- It is buried in shallow landfill sites.
- It can also be compacted to reduce its volume and burnt in a closed container before disposal.
Intermediate-level Waste
- It makes up 7% of the volume and 4% of the radioactivity of all radioactive waste.
- It comprises resins, chemical sludges, reactor components and contaminated materials from reactor decommissioning.
- It often contains higher amounts of radioactivity and may require special shielding.
- It can be solidified in concrete or bitumen for disposal.
- Short-lived waste (mainly from reactors) is buried at isolated sites which are geologically stable.
- Long-lived waste (from reprocessing nuclear fuel) is disposed off deep underground.
High-level Waste
- It consists of only 3% of the volume of all radioactive waste but it holds 95% of the radioactivity.
- It is mainly used fuel rods or liquid waste from fuel reprocessing.
- It still contains highly-radioactive fission products and some heavy elements with very long half-lives.
- It generates large amounts of heat and requires cooling as well as special shielding during handling and transport.
- It is vitrified by incorporating it into borosilicate glass which is then sealed inside stainless steel canisters for eventual disposal deep underground.
