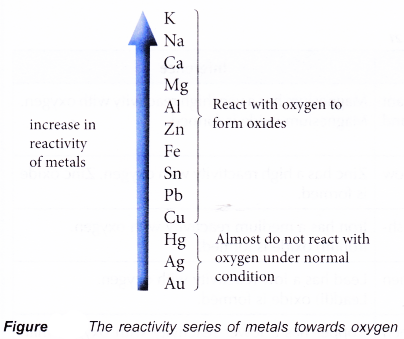
The Reactivity Series of Metals Towards Oxygen
- The reactivity of metals differs from one metal to another. In fact, the form in which a metal occurs in nature depends on its reactivity.
- Gold has very low reactivity and therefore can be found in its metallic state in nature.
- Aluminium, potassium and sodium have very high reactivity, and therefore exist as compounds in nature.
- One of the common compounds formed by metals is metal oxide. The formation of metal oxide is a redox reaction.
Metal + oxygen → metal oxide
(a) Metal undergoes oxidation to form positive ions. Its oxidation number increases from zero to a positive value.
(b) Oxygen undergoes reduction to form oxide ions. Its oxidation number decreases from 0 to -2.
(c) Thus, metal acts as the reducing agent while oxygen acts as the oxidising agent in the formation of metal oxide. - The more reactive a metal is towards oxygen, the more vigorously it burns in oxygen.
- Hence, by observing how vigorously the metals react with oxygen, we can arrange the metals according to their reactivity towards the oxygen.
- The reactivity series of metals towards oxygen is a list of metals according to their reactivity with oxygen. This series of metals is quite similar to the electrochemical series because the reactivity of a metal with oxygen is closely linked to its ability to lose electrons.
People also ask
- What is a redox reaction?
- Changing of iron(II) ions to iron(III) ions and vice versa
- Redox reaction in the displacement of metals from its salt solution
- Displacement of Halogen From Halide Solution
- Redox Reactions by Transfer of Electrons at a Distance
- Rusting as a Redox Reaction
- Application of the reactivity series of metals in the extraction of metals
- Electrolytic and Chemical Cells
- Oxidation and Reduction in Electrolytic Cells
- Oxidation and Reduction in Chemical Cells
- How does a voltaic cell work?
Reactivity Series of Metals with Oxygen Experiment
Aim: To investigate the reactivity of metals with oxygen.
Materials: Magnesium powder, zinc powder, iron filings, lead powder, copper powder, solid potassium manganate(VII), asbestos paper, glass wool.
Apparatus: Boiling tube, retort stand and clamp, Bunsen burner, spatula, forceps.
Safety measure:
Asbestos paper and glass wool are hazardous and should be handled with care.
Procedure:
- One spatulaful of solid potassium manganate(VII) is put into a boiling tube.
- Some glass wool is pushed into the tube. The tube is clamped horizontally as shown in Figure.
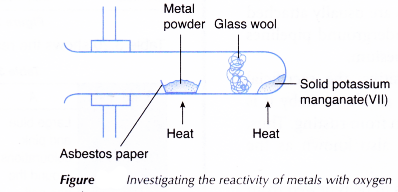
- One spatulaful of magnesium powder is placed on a piece of asbestos paper and is put into the tube.
- The magnesium powder is heated strongly. Then, the solid potassium manganate(VII) is heated. How vigorously magnesium reacts with oxygen and the colour of the residue when it is hot and when it is cold are observed.
- Steps 1 to 4 are repeated using zinc powder, iron filings, lead powder and copper powder, one at a time, in place of magnesium powder.
Results:
| Metal | Observation | Inference |
| Magnesium | Magnesium burns brightly with a very brilliant white flame. The residue is white when hot and cold. | Magnesium has a very high reactivity with oxygen. Magnesium oxide is formed. |
| Zinc | Zinc burns fairly bright. The residue is yellow when hot and white when cold. | Zinc has a high reactivity with oxygen. Zinc oxide is formed. |
| Iron | Iron glows very brightly. The residue is reddish- brown when hot and cold. | Iron has a medium reactivity with oxygen. Iron(III) oxide is formed. |
| Lead | Lead glows brightly. The residue is brown when hot and yellow when cold. | Lead has a low reactivity with oxygen. Lead(II) oxide is formed. |
| Copper | Copper glows faintly. The residue is black when hot and cold. | Copper has a lower reactivity with oxygen than lead. Copper(II) oxide is formed. |
Discussion:
- When solid potassium manganate(VII) is heated, it decomposes to give out oxygen gas.
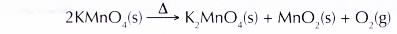
- Other than solid potassium manganate(VII), oxygen gas can also be provided by:
(a) Heating a mixture of potassium chlorate(V) with manganese(IV) oxide as a catalyst
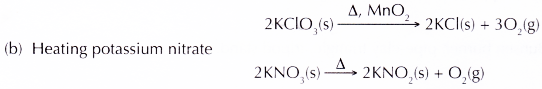
- The glass wool separates the metal powder from the solid potassium manganate(VII). If the substances are mixed, the mixture of metal powder and solid potassium manganate(VII) will explode when heated.
- Based on the vigour of the reactions, the metals can be arranged according to their reactivity with oxygen.

- The following equations represent the reactions between the metals and oxygen.
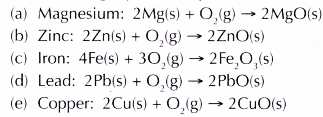
Conclusion:
The descending order of reactivity of metals with oxygen is Mg, Zn, Fe, Pb, Cu.
Position of Carbon in the Series of Reactivity of Metals
- The position of carbon in the series can be determined based on:
(a) The ability of carbon to remove oxygen from metal oxides
(b) The ability of metals to remove oxygen from carbon dioxide - Ability of carbon to remove oxygen from metal oxides
(a) Carbon is strongly heated with a metal oxide.
(b) If carbon is more reactive than the metal, it can remove oxygen from the metal oxide. In other words, carbon can reduce the metal oxide to metal.
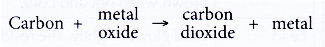
(c) Conversely, if carbon is less reactive than the metal, it cannot remove oxygen from the metal oxide. - Ability of metals to remove oxygen from carbon dioxide
(a) A heated metal is placed in carbon dioxide.
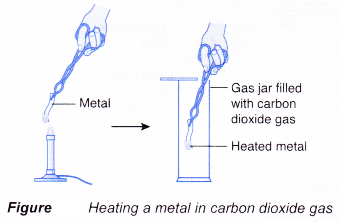
(b) If the metal is more reactive than carbon, the metal can remove oxygen from carbon dioxide. In other words, the metal can reduce carbon dioxide to carbon.
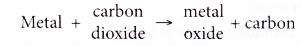
(c) On the other hand, if the metal is less reactive than carbon, it is unable to remove oxygen from carbon dioxide.
(d) (i) For example, when a piece of magnesium ribbon is heated and placed in a gas jar filled with carbon dioxide, the magnesium ribbon burns brightly, producing a white residue of magnesium oxide. A lot of black powder of carbon is also formed on the wall of the gas jar.
(ii) The redox reaction can be represented as follows.
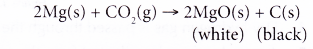
As magnesium is more reactive than carbon, it is able to remove oxygen from carbon dioxide. Magnesium reduces carbon dioxide to carbon and magnesium itself is oxidised to magnesium oxide.
Position of Carbon in the Series of Reactivity of Metals Experiment
Aim: To determine the position of carbon in the reactivity series of metals towards oxygen.
Materials: Carbon powder, solid copper(II) oxide, solid magnesium oxide, solid aluminium oxide, solid zinc oxide.
Apparatus: Crucible, spatula, Bunsen burner, pipe-clay triangle, tripod stand.
Procedure:
- A spatulaful of carbon powder and a spatulaful of solid copper(II) oxide are mixed thoroughly in a crucible.
- The apparatus is set up as shown in Figure.
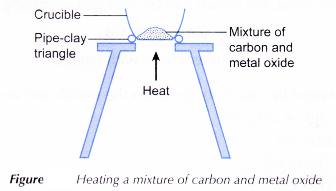
- The mixture is heated strongly. Any changes that occur are observed.
- Steps 1 to 3 are repeated using solid zinc oxide, solid aluminium oxide and solid magnesium oxide, one at a time, in place of solid copper(II) oxide.
Results:
| Mixture | Observation | Inference |
| Carbon + copper(II) oxide | A flame spreads to the whole mixture. A brown residue is formed. | Metallic copper is formed. Carbon has reduced copper(II) oxide to copper. Thus, carbon is more reactive than copper. |
| Carbon + zinc oxide | A glow spreads to the whole mixture. A grey residue is formed. | Metallic zinc is formed. Carbon has reduced zinc oxide to zinc. Thus, carbon is more reactive than zinc. |
| Carbon + aluminium oxide | No change | Carbon is unable to reduce aluminium oxide. Thus, carbon is less reactive than aluminium. |
| Carbon + magnesium oxide | No change | Carbon is unable to reduce magnesium oxide. Thus, carbon is less reactive than magnesium. |
Discussion:
- Carbon is more reactive than copper and zinc. Therefore, carbon can reduce copper(II) oxide and zinc oxide to their respective metals.

- Carbon is less reactive than aluminium and magnesium. Thus, carbon is unable to reduce aluminium oxide and magnesium oxide.
Conclusion:
Carbon is positioned between aluminium and zinc in the reactivity series of metals towards oxygen.
![]()
Position of Hydrogen in the Reactivity Series of Metals
- We can determine the position of hydrogen in the series based on the ability of hydrogen to remove oxygen from metal oxides.
- To do this, a metal oxide is heated in the presence of hydrogen.
(a) If hydrogen is more reactive than the metal, hydrogen is able to remove the oxygen from the metal oxide. In other words, hydrogen is able to reduce the metal oxide to its metal while hydrogen itself is oxidised to water.
Hydrogen + metal oxide → metal + water
(b) On the other hand, if hydrogen is less reactive than the metal, hydrogen is unable to remove the oxygen from the metal oxide. Thus, no reaction will take place. - Using the same way, we can predict the position of other metals in the reactivity series.
- The reactivity series of metals towards oxygen can assist us in predicting reactions involving metals.
(a) Reaction of metal oxides with carbon or hydrogen
Carbon + metal oxide → metal + carbon dioxide
Hydrogen + metal oxide → metal + water
A reaction will take place if carbon or hydrogen is more reactive than the metal. The carbon or hydrogen will remove the oxygen from the metal oxide.
(b) Reaction of metal oxides with other metals
Metal X + oxide of metal Y → oxide of metal X + Metal Y
This reaction occurs if metal X is more reactive than metal Y.
(c) Reaction of metals with water or steam.
Metal + water/steam → metal oxide + hydrogen
This reaction occurs if the metal is more reactive than hydrogen.
(d) Reaction of metals with carbon dioxide
Metal + carbon dioxide → metal oxide + carbon
This reaction occurs if the metal is more reactive than carbon.
Position of Hydrogen in the Reactivity Series of Metals Experiment
Aim: To determine the position of hydrogen in the reactivity series of metals towards oxygen.
Materials: 2 mol dm-3 sulphuric acid, 1 mol m-3 copper(II) sulphate solution, zinc granules, solid copper(II) oxide, solid zinc oxide, solid lead(II) oxide, solid iron(III) oxide, anhydrous calcium chloride.
Apparatus: Combustion tube, porcelain dish, flat-bottomed flask, U-tube, thistle funnel, delivery tubes, Bunsen burner, retort stand and clamps, stoppers with one hole, stopper with two holes.
Safety measure:
A mixture of hydrogen and air will explode when lighted.
Ensure that the flow of hydrogen is continuous throughout the activity.
Procedure:
- A spatulaful of solid copper(II) oxide is placed in a porcelain dish.
- The porcelain dish is placed in a combustion tube and the tube is clamped horizontally.
- The apparatus is set up as shown in Figure.
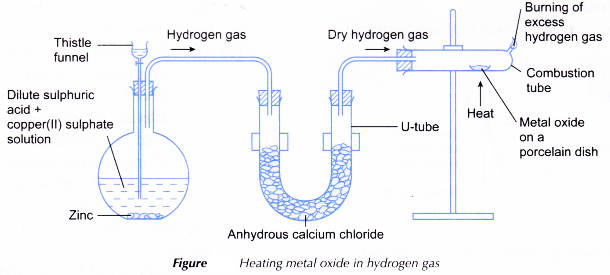
- Dry hydrogen gas is passed through the combustion tube for 5 to 10 minutes to remove all the air in the tube.
- A sample of gas is collected from the small hole at the end of the combustion tube.
- The gas is tested with a lighted wooden splint.
- If the gas burns quietly without a squeaky ‘pop’, all the air in the tube has been removed. Otherwise, steps 5 and 6 are repeated until all the air in the tube has been removed.
- The excess hydrogen gas that comes out of the end of the combustion tube is lighted.
- Solid copper(II) oxide is strongly heated. Any change is observed. The flow of hydrogen gas should be continuous throughout this activity.
- Steps 1 to 9 are repeated using solid zinc oxide, solid lead(II) oxide and solid iron(III) oxide, one at a time, in place of solid copper(II) oxide.
Results:
Discussion:
- Hydrogen gas is produced when the zinc granules react with sulphuric acid with the presence of copper(II) sulphate solution as a catalyst.
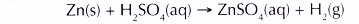
The hydrogen gas produced is dried by passing it through anhydrous calcium chloride. Another drying agent that can be used is concentrated sulphuric acid. - The following precautions must be taken to prevent any explosion from happening.
(a) All connections to delivery tubes and stoppers should be tight.
(b) All the air in the combustion tube must be removed before lighting up the hydrogen gas that comes out of the end of the combustion tube. Otherwise, a mixture of hydrogen and air will explode when lighted.
(c) The flow of hydrogen gas should be continuous throughout the activity. - Hydrogen is more reactive than copper, lead and iron. Therefore, hydrogen can reduce copper(II) oxide, lead(II) oxide and iron(III) oxide to their respective metals.

- Hydrogen is less reactive than zinc. Therefore, hydrogen is unable to reduce zinc oxide.
Conclusion:
Hydrogen is positioned between zinc and iron in the reactivity series of metals towards oxygen.
![]()
