Radioactivity: Types of Radioactive Emissions
What is radioactivity in physics?
Radioactivity:
- You may have heard of this statement
“Danger : Radiation Kills”. Do you know that radiation can kill for a good purpose?

- The syringe and needle used by a doctor is cleaned by gamma radiation. What is radiation and how does it kill bacteria and other organisms?
- In 1896, the French scientist Henri Becquerel discovered that some uranium salts give out invisible rays that can go through thick paper and darken a photographic plate.
- These rays or radiation are given out by the nuclei of the uranium atoms.
- The nuclei of some atoms are unstable. An unstable nucleus will decay to become more a stable nucleus by emitting radiation, in the form of a particle or electromagnetic radiation.
- Radioactivity is the spontaneous disintegration of an unstable nucleus accompanied by the emission of an energetic particle or a photon.
- Figure gives a diagrammatic representation of a radioactive disintegration.

- The energetic particle or photon is also known as radioactive emission.
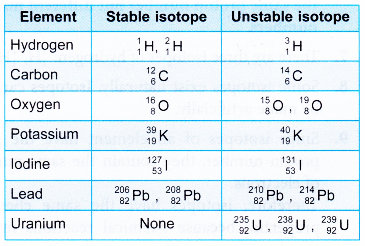
- The atoms of an element can exist as isotopes. Some isotopes have nuclei that are stable while some isotopes have nuclei that are unstable.
- Stable nuclei remain as they are and do not undergo any changes.
- Unstable nuclei will undergo radioactive disintegration or radioactive decay to become more stable.
- Table gives some examples of stable and unstable isotopes.
People also ask
- How do you detect radioactivity?
- What are the different types of radioactive decay?
- What are the Isotopes, Isobars and Isotones of an Element
- What is the Radioactive Isotope?
- Importance of Proper Management of Radioactive Substances
- What is nucleus of an atom?
- What is the half life of a radioactive element?
- What is Nuclear Energy?
- What is nuclear fission and how does it occur?
- How is energy released in a nuclear fusion reaction?
- What happens in a nuclear chain reaction?
- How does a nuclear power plant works?
List three types of radiation that are produced during radioactivity
Types of Radioactive Emissions:
- There are three kinds of radioactive emissions:
(a) Alpha particles (α-particles)
(b) Beta particles (β-particles)
(c) Gamma rays (γ-rays) - Alpha particles, beta particles and gamma rays can be represented diagramatically as in Figure 5.9:
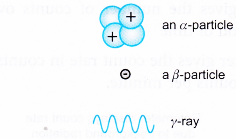
- An alpha particle:
(a) is a helium nucleus
(b) consists of two protons and two neutrons
(c) is positively charged
(d) is very heavy compared to a beta particle
(e) moves slower than a beta particle; up to about 10% of the speed of light - A beta particle:
(a) is a high energy electron
(b) is negatively charged
(c) is very much lighter than an alpha particle
(d) moves at very high speed; up to 99% of the speed of light - Gamma rays:
(a) are electromagnetic waves
(b) have very high frequencies and short wavelengths (10-13m < λ < 10-11 m)
(c) do not carry any charge
(d) move at the speed of light in a vacuum
Characteristics of Radioactive Emissions
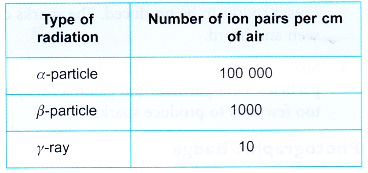
- Alpha particles, beta particles and gamma rays ionise the atoms of the medium through which they pass to produce ion pairs.
- Alpha particles have a relatively larger mass and are positively-charged. They have the highest ionising power and produce the most ion pairs in a medium.
- Beta particles have a much smaller mass and are negatively-charged. They have a lower ionising power than a-particles.
- Gamma rays are electromagnetic waves and have the lowest ionising power.
- The ionisation power of α, β and γ is indicated by the number of ion pairs produced per cm travelled in air as shown in Table.
- The radioactive emission loses some of its energy each time an ion pair is produced.
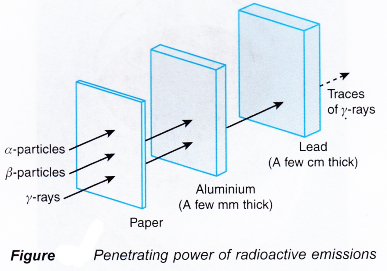
- Alpha particles which have the highest ionising power lose energy relatively quickly as they move through a medium. After a short distance in the medium, the alpha particles would have lost almost all of its energy. Therefore, alpha particles have the lowest penetrating power.
- Gamma rays which have the lowest ionising power, have the highest penetrating power.
- Figure shows the approximate range of the three radioactive emissions in air.

- Figure compares the penetrating powers of different radioactive emissions.
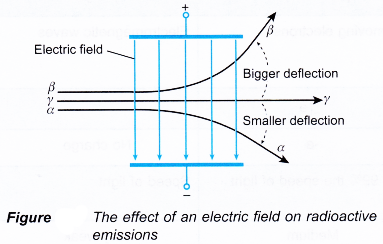
- Alpha and beta particles are deflected in an electric field because they are charged. The deflections are in opposite directions because they carry opposite charges.
- However, the alpha particles go through a smaller deflection than the beta particles as their mass is much larger.
- Figure compares the paths of radioactive emissions in an electric field. Gamma rays are not deflected because they do not carry any charge. Note that the paths of the alpha and beta particle are curved only in the region between the plates.
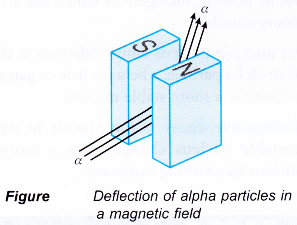
- Figure shows the effect of a magnetic field on alpha particles.
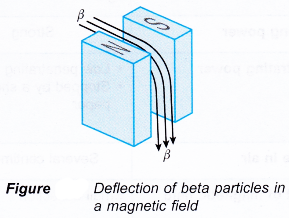
- Alpha particles and beta particles are deflected when they pass through a magnetic field while γ-rays are unaffected. Alpha particles and beta particles follow circular paths in a magnetic field.
- The direction of the deflection of alpha particles in a magnetic field can be found using Fleming’s Left Hand Rule where the
- First finger: Direction of the magnetic field
- Second finger: Direction of motion of the alpha particles
- Thumb: Direction of deflection
- Beta particles are deflected in the opposite direction along a circular path with a smaller radius, as shown on Figure.
- Figure shows the paths taken when a beam consisting of alpha particles, beta particles and gamma rays move in a magnetic field.
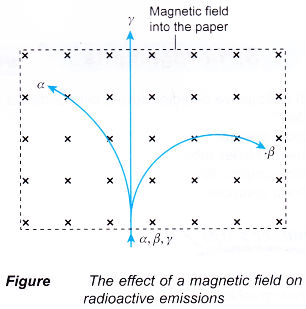
Table summarises the nature and characteristics of the three types of radioactive emissions.
| Properties | α-particles | β-particles | γ-rays |
| Nature |
| Fast moving electrons | Electromagnetic waves |
| Symbol | 2He4 | -1e0 | – |
| Charge | +2e | -e | No charge |
| Speed | Up to 10% the speed of light | Up to 99% the speed of light | Speed of light |
| Ionising power | Strong | Medium | Very weak |
| Penetrating power |
|
|
|
| Range in air | Several centimetres | Several metres | Several hundred metres |
| Effect of magnetic field | Small deflection | Larger deflection in the opposite direction of the a-particles | No deflection |
| Effect of electric field | Small deflection towards a negatively-charged plate | Larger deflection towards a positively-charged plate | No deflection |
| Tracks in cloud chamber | Straight and thick lines | Thin and wavy lines | Very fine short lines |
