What are the properties of a pure metal?
Pure Metals:
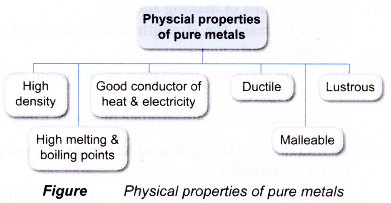
- Typical pure metals have the following physical properties:
- Ductile- can be drawn into fine wire
- Malleable- can be beaten into thin sheets without cracking
- Lustrous- becomes shiny when polished
- High density
- High melting and boiling points
- Good conductor of heat and electricity
- These properties of pure metals are reflected by their arrangement of atoms.
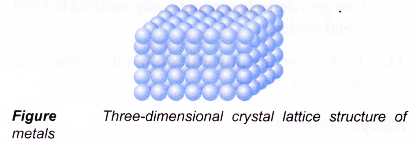
- Pure metal is made up of only one type of atom, thus the atoms are of the same size.
- Since all the atoms are of the same size, in solid state, these atoms are orderly arranged and closely packed together. Thus pure metals have high density.
- The atoms in metal are orderly arranged in layers to form three-dimensional crystal lattice as shown in Figure.
- Metals have high melting and boiling points due to the existence of very strong forces of attraction between the closely-packed atoms. Thus more heat energy is required to overcome the forces of attraction.
- Although the forces of attraction between the atoms are strong, pure metals are weak and soft due to their ductility and malleability and have limited use.
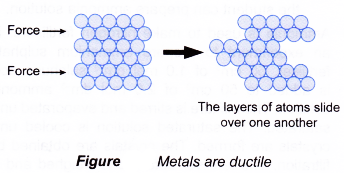
- Pure metals are ductile and soft because all the atoms are of the same size and orderly arranged, thus the layers of atoms can slide over one another easily when a force is applied.
- Pure metals are malleable, weak and can be shaped. This is because there are some empty spaces in between the atoms of pure metals. When a metal is knocked or pressed, groups of atoms may slide and settle into new positions due to the imperfection.
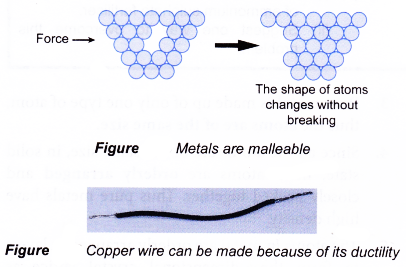
- Because pure metals are ductile and malleable, they are relatively soft and easily oxidised to form metal oxides.
- To improve the properties of a pure metal, it is made into alloy.
