Properties of Ionic and Covalent Compounds
- Ionic and covalent compounds differ in their properties because the particles in each of these two compounds are held together by different types of chemical bonds.
- Table compares and contrasts the properties of ionic and covalent compounds.
Covalent compounds Ionic compounds (composed of simple molecules) (a) Have high melting and boiling points (a) Have low melting and boiling points (b) Exist as solids at room temperature.
Non-volatile(b) Usually exist as liquids or gases at room temperature.
Volatile(c) Conduct electricity in the molten state or in an aqueous solution but do not conduct electricity in the solid state
(c) Do not conduct electricity in the solid and liquid states (d) Usually soluble in water but insoluble in organic solvents such as ether, alcohol, benzene, tetrachloromethane, propanone and other (d) Usually insoluble in water but soluble in organic solvents such as ether, alcohol, benzene, tetrachloromethane, propanone and other
Explaining the melting and boiling points of ionic compounds
- Table shows the melting and boiling points of some ionic compounds.
Ionic compound Melting point (°C) Boiling point (°C) Calcium oxide, CaO 2580 2850 Magnesium chloride, MgCl2 714 1412 Sodium fluoride, NaF 993 1695 Aluminium oxide, Al2O3 2030 2970 Sodium chloride, NaCl 801 1420 - The melting and boiling points of ionic compounds are high.
- Table shows the melting and boiling points of some ionic compounds.
- The high melting and boiling points of ionic compounds can be explained as below:
- Ionic compounds are composed of oppositely-charged ions (positive and negative ions) arranged in a three-dimensional giant crystal lattice.
- The oppositely-charged ions are held together by strong electrostatic forces of attraction, known as ionic bonds.
- A lot of heat energy is needed to break the strong ionic bonds during melting or boiling.
- Hence, ionic compounds have high melting and boiling points with low volatility.
People also ask
- Chemical Bonding and Compound Formation
- Chemical Bonding
- What is Covalent Bond?
- How is covalent bond is formed?
- Describe how to write a formula for a covalent compound
- What causes ions to form ionic bonds?
- Explain the formation of ionic bonds with examples
- How do you write the formula for ionic compounds?
- How do you Name an Ionic Compound?
Explaining the melting and boiling points of covalent compounds
- Covalent compounds are composed of molecules.
- The bonding in these covalent compounds consists of
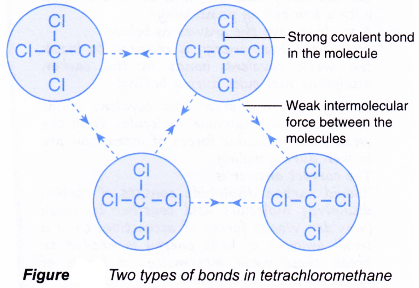
- strong covalent bonds between the atoms in the molecule.
- weak forces of attraction between the molecules.
- An example is shown in Figure. It shows the two types of bonds in liquid tetrachloromethane, CCl4.
- Table shows the melting and boiling points of four covalent compounds.
Covalent compound Melting point
(°C)Boiling point
(°C)Ethanol, C2H5OH -117 78 Tetrachloromethane, CCl4 -23 76.8 Ammonia, NH3 -78 -33 Methane, CH4 -182 -164 - The melting and boiling points of covalent compounds are low.
- Table shows the melting and boiling points of four covalent compounds.
- The low melting and boiling points of covalent compounds can be explained as below:
- In a covalent compound, the covalent molecules are held together by weak forces of attraction.
- A small amount of heat energy is required to overcome the weak intermolecular forces of attraction during melting or boiling.
- Hence, the covalent compound has low melting and boiling points with high volatility.
Explaining the electrical conductivity of ionic compounds
- The electrical conductivity of ionic compounds in the solid state can be explained as below:
- Ionic compounds are composed of oppositely-charged ions.
- In the solid state, the positive and negative ions are locked in fixed positions and cannot move freely.
- Hence, ionic compounds cannot conduct electricity in the solid state.
- The electrical conductivity of ionic compounds in the molten (liquid) and aqueous states can be explained below:
- When the ionic compounds are melted through heating or dissolved in water, the positive and negative ions will break free and become mobile, that is able to move freely.
- The presence of free mobile ions enable ionic compounds to conduct electricity in the molten or aqueous states.
Explaining the electrical conductivity of covalent compounds
- Table shows the electrical conductivity of a few covalent compounds.
Covalent compound Electrical conductivity Solid Liquid Glucose Non conductor Non conductor Acetamide Non conductor Non conductor Napthalene Non conductor Non conductor Tetrachloromethane Non conductor Non conductor - The electrical conductivity of covalent compounds in the solid and liquid states can be explained as below:
- Covalent compounds are composed of simple covalent molecules in the solid and liquid states.
- There are no free mobile ions in these two states.
- Hence, covalent compounds cannot conduct electricity in the solid and liquid states.
Explaining the solubility of ionic compounds
- The solubility of ionic compounds in water can be explained as below:
- Ionic compounds are composed of ions.
- The ions are easily hydrated by water molecules to form hydrated ions.
- The hydration of ions by water molecules liberates heat energy.
- As a result, ionic compounds are usually soluble in water.
- The solubility of ionic compounds in organic solvents can be explained as below:
- Organic solvents such as ether, alcohol, benzene and tetrachloromethane consist of covalent molecules which cannot hydrate ions.
- As a result, ionic compounds are insoluble in organic solvents.
Explaining the solubility of covalent compounds
- The solubility of covalent compounds in water can be explained as below:
- Covalent compounds consist of covalent molecules.
- Water cannot hydrate covalent molecules.
- Hence, covalent compounds are usually insoluble in water.
- The solubility of covalent compounds in organic solvents can be explained as below:
- Covalent molecules in covalent compounds and organic molecules in organic solvents are both held together by weak intermolecular forces of attraction.
- As a result, the covalent molecules in the covalent compounds are easily miscible with the organic molecules in the organic solvents because they have the same type of weak intermolecular forces of attraction.
- Hence, covalent compounds are usually soluble in organic solvents.
Properties of Ionic and Covalent Compounds Experiment
Aim: To compare the properties of ionic and covalent compounds.
Materials: Magnesium chloride crystals, sodium sulphate crystals, solid lead(II) bromide, diethyl ether, hexane, cyclohexane, distilled water and naphthalene.
Apparatus: Watch glasses, dropper, test tubes, crucible, battery, bulb, switch, Bunsen burner, tripod stand, carbon electrodes, pipe-clay triangles, connecting wires with crocodile clips and beaker.
Procedure:
A. Melting and boiling points
- Half spatula of magnesium chloride crystals and sodium sulphate crystals are placed separately in two different watch glasses. The physical state of each substance is recorded.
- Three drops of diethyl ether and hexane are placed separately in two different watch glasses. The physical state of each substance is recorded.
- All the watch glasses are left aside for 5 to 10 minutes. All the changes are recorded.
- Inferences regarding their volatility, melting and boiling points are made based on the observation.
B. Solubility in water and organic solvents
- A quarter spatula of magnesium chloride crystals are placed in a test tube.
- 5 cm3 of distilled water is added to the test tube.
- The mixture in the test tube is shaken well.
- All the changes are recorded.
- Steps 1 to 4 are repeated using liquid cyclohexane to replace distilled water.
- Steps 1 to 5 are repeated using 5 cm3 of diethyl ether to replace the magnesium chloride crystals.
C. Electrical conductivity
- A crucible is filled with solid lead(II) bromide until it is half full.
- The apparatus as shown in Figure is set up.
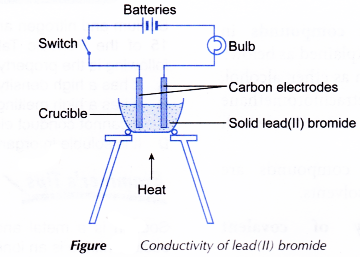
- The switch is turned on. The observation on whether the bulb glows and the changes at the electrodes (if any) are made.
- The switch is then turned off. The solid lead(II) bromide in the crucible is heated until it melts completely.
- The switch is turned on again. The observation on whether the bulb glows and the changes at the electrodes (if any) are made.
- Steps 1 to 5 are repeated using solid naphthalene to replace solid lead(II) bromide.
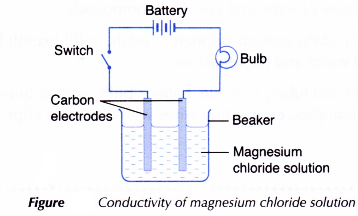
- Another test on the electrical conductivity of aqueous magnesium chloride solution is carried out by setting up the apparatus as shown in Figure. Observation on whether the bulb glows and the changes at the electrodes (if any) are recorded.
Results:
A. Melting and boiling points
| Substance | Observation | Inferences |
| Magnesium chloride crystals | The substance remains as a white solid even after 10 minutes. | Magnesium chloride has high melting and boiling points. It is non-volatile. |
| Sodium sulphate crystals | The substance remains as a white solid even after 10 minutes. | Sodium sulphate has high melting and boiling points. It is non-volatile. |
| Diethyl ether | The colourless liquid disappears/vaporises and the watch glass becomes dry after 10 minutes. | Diethyl ether has low melting and boiling points. It is volatile. |
| Hexane | The colourless liquid disappears/vaporises and the watch glass becomes dry after 10 minutes. | Hexane has low melting and boiling points. It is volatile. |
B. Solubility in water and organic solvents
| Substance | Observation | Inferences | |
| Solubility in water | Solubility in cyclohexane | ||
| Magnesium chloride | The white solid dissolves in water to form a colourless solution. | The white solid does not dissolve in cyclohexane. | Magnesium chloride is soluble in water but insoluble in cyclohexane. |
| Diethyl ether | Two layers of colourless liquids are formed. | The colourless liquid dissolves in cyclohexane to form a colourless solution. | Diethyl ether is insoluble in water but soluble in cyclohexane. |
C. Electrical conductivity
| Substance | State of substance | Observation | Inferences | |
| The bulb | Changes at the carbon electrodes | |||
Lead(II) bromide | Solid | The bulb does not glow. | No change | Lead(II) bromide cannot conduct electricity in the solid state but can conduct electricity in the liquid state. |
| Liquid/molten | The bulb glows brightly. | A reddish-brown vapour is liberated at one of the electrodes. | ||
| Naphthalene | Solid | The bulb does not glow. | No change | Naphthalene cannot conduct electricity in the solid and liquid states. |
| Liquid/molten | The bulb does not glow. | No change | ||
| Magnesium chloride | Aqueous solution | The bulb glows brightly. | Bubbles of gas are liberated at both the carbon electrodes. | Magnesium chloride can conduct electricity in the aqueous solution. |
Discussion:
- Magnesium chloride crystals and sodium sulphate crystals are ionic compounds. They are made up of positive and negative ions which are attracted together by strong ionic bonds. A lot of heat energy is needed to overcome these bonds during melting or boiling. Hence, they have high melting and boiling points and are non-volatile.
- Diethyl ether and hexane are covalent compounds. They consist of molecules that are attracted to each other by weak intermolecular forces. Little heat energy is needed to overcome these weak forces during melting or boiling. Hence, they have low melting and boiling points and are volatile.
- Magnesium chloride, as an ionic compound, is
- soluble in water, but
- insoluble in cyclohexane (organic solvent).
- Diethyl ether, as a covalent compound, is insoluble in water, but soluble in cyclohexane (organic solvent).
- In solid lead(II) bromide (an ionic compound), the lead(II) ions and bromide ions are closely packed at fixed positions in an orderly manner. Hence, the ions do not move freely. As a result, solid lead(II) bromide cannot conduct electricity.
- In molten lead(II) bromide, the lead(II) ions and bromide ions are mobile or can move freely. Hence, molten lead(II) bromide can conduct electricity.
- Magnesium chloride (an ionic compound) ionises completely in an aqueous solution to become free mobile magnesium ions and chloride ions. Hence, an aqueous solution of magnesium chloride can conduct electricity.
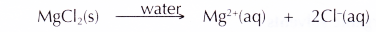
- Naphthalene, as a covalent compound, is made up of covalent molecules only. Hence, it cannot conduct electricity in the solid and liquid states.
Conclusion:
- Ionic compounds are non-volatile and have high melting and boiling points. They are usually soluble in water but insoluble in organic solvents. They can conduct electricity in the molten and aqueous states.
- Covalent compounds are volatile and have low melting and boiling points. They are usually insoluble in water but soluble in organic solvents. They cannot conduct electricity in the solid and liquid states.
Types of covalent molecules
- There are two types of covalent molecules.
(a) Simple molecules such as water, carbon dioxide, ammonia and tetrachloromethane.
(b) Macromolecules or giant molecules such as silicon dioxide and diamond. - Figure shows the structures of diamond and silicon dioxide.
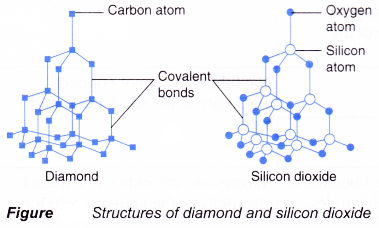
- In a macromolecule, all the atoms are bonded to each other by covalent bonds in a giant lattice structure.
- These macromolecules
(a) have high melting and boiling points because a lot of heat energy is needed to break the strong covalent bonds in the giant lattice structure.
(b) cannot conduct electricity because they do not have free mobile ions.
(c) are insoluble in water.
Uses of covalent compounds as solvents
- Many covalent compounds have low melting and boiling points. Hence, they exist as liquids at room temperature and are volatile.
- Covalent compounds in the form of liquids are mostly used as solvents in our daily life. Most of these liquids are organic compounds. They are known as organic solvents.
- Examples of some common organic solvents are alcohols such as ethanol, ethers such as dimethyl dimethyl ether, propanone, chloroform (trichloromethane), turpentine and petrol.
- Organic solvents are used
(a) as solvents to prepare solutions.
(b) to remove and clean dirt on surfaces which cannot be removed by water. - Table lists out the uses of some organic solvents.
Solvents Uses Turpentine To dissolve paint Petrol and kerosene As solvents to remove greasy or oil dirts Alcohols, propanone and turpentine As solvents to prepare varnish, shellac and lacquer Alcohols As solvents in medicine such as iodine solution Ethers As solvents in the extraction of chemicals from aqueous solutions Propanone To remove nail varnish Chlorofluorocarbons
(CFC)As solvents to clean computer circuit boards Alcohols and ethers As solvents for ink and dyes Alcohols, ethers and propanone As volatile solvents in the preparation of cosmetic products such as perfumes - Most organic solvents such as benzene, chloroform and propanone are poisonous and harmful.
