What is the Process of Electrolysis
Electrolysis
The production of a chemical reaction by passing an electric current through an electrolyte is called electrolysis.
We know that an electrolyte contains ions, which are charged. The positively charged ions are called cations, because they are attracted to the cathode, and the negatively charged ones are called anions, because they are attracted to the anode. We know that unlike charges attract and like charges repel. Cations, being positively charged, get attracted to the negatively charged cathode and move towards it. Anions, being negatively charged, get attracted to the positively charged anode and move towards it. This explains how ions move in an electrolytic cell, and thus ‘conduct’ an electric current.
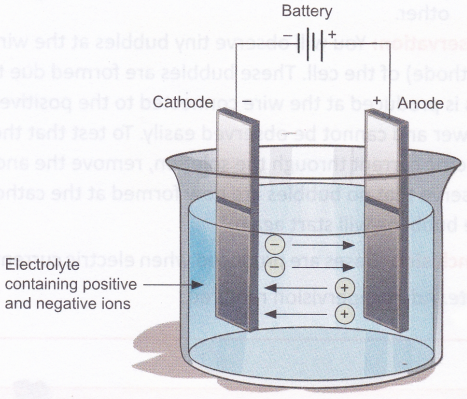
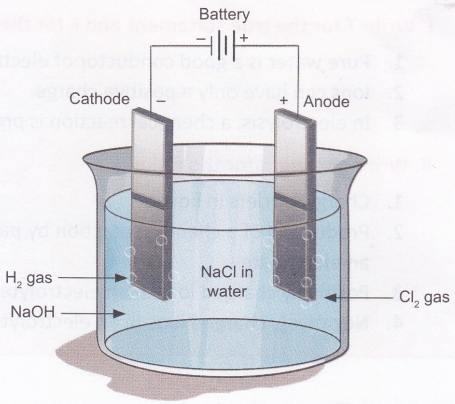
A chemical reaction takes place at the anode and the cathode. This can be observed as formation of bubbles (due to production of gases) or deposition of metal on the electrodes or as a change in the colour of the electrolyte. The reaction varies depending on the metals used for the electrodes and the electrolyte chosen. Electrolysis of a solution of sodium chloride (NaCl) produces hydrogen gas (H2), chlorine gas (Cl2), and sodium hydroxide (NaOFI).
Activity
Aim: To show that gases are produced when electric current is passed through a solution of common salt.
Materials needed: A 9-volt cell, two electrical wires (about 10 cm each), a beaker/glass tumbler (if a glass tumbler is used it should be discarded after the experiment), insulation tape, a blade, tap water, and table salt.
Method:
1. Take the two wires and remove the jacket from each end using a blade.
2. Connect one end each of the two wires to the positive and negative terminals of the cell.
3. Fill the tumbler with tap water and dissolve common salt in it.
4. Dip the two free ends of the wires into the water, taking care that they do not touch each other.
Observation: You will observe tiny bubbles at the wire connected to the negative terminal (cathode) of the cell. These bubbles are formed due the formation of hydrogen gas. Chlorine gas is produced at the wire connected to the positive terminal (anode), but the reaction is much slower and cannot be observed easily. To test that the bubbles are indeed formed on passing electric current through the solution, remove the anode wire from the salt solution. You will observe that no bubbles are now formed at the cathode. Dip the wire back into the solution and the bubbling will start again.
Conclusion: Gases are produced when electric current is passed through a solution of common salt.
Note: Adult supervision required.
