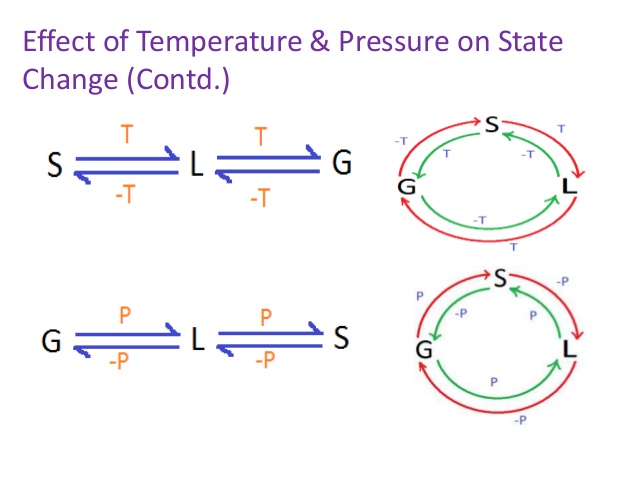
How does Pressure Affect Changes of State
Effect of Change of Pressure
- The three states of matter differ in the intermolecular forces and intermolecular distances between the constituent particles.
- Gases are compressible because on applying pressure, the space between the gaseous particles decreases. Therefore, gases can be compressed readily.
- When we apply pressure and reduce temperature the gases can be converted into liquids i.e., gases will be liquefied.
- The process of conversion of a gas into a liquid by increasing pressure or decreasing temperature is called liquefication.
A substance may exist in any of the three different states of matter depending upon the conditions of temperature and pressure.
- If the melting point of a substance is above the room temperature at the atmospheric pressure, it is said to be a solid.
- If the boiling point of a substance is above room temperature under atmospheric pressure, it is classified as liquid.
- If the boiling point of the substance is below the room temperature at the atmospheric pressure, it is called a gas.