Kerala Plus Two Chemistry Previous Year Question Paper March 2018 with Answers
| Board | SCERT |
| Class | Plus Two |
| Subject | Chemistry |
| Category | Plus Two Previous Year Question Papers |
Time: 2 Hours
Cool off time: 15 Minutes
Maximum: 60 Score
General Instructions to candidates:
- There is a ‘cool off time’ of 15 minutes in addition to the writing time of 2 hrs.
- Use the ‘cool off time’ to get familiar with the questions and to plan your answers.
- Read questions carefully before you answering.
- Read the instructions carefully.
- Calculations, figures, and graphs should be shown in the answer sheet itself.
- Malayalam version of the questions is also provided.
- Give equations wherever necessary.
- Electronic devices except non-programmable calculators are not allowed in the Examination Hall.
(Questions 1 to 7): Carry one score each. Answer all questions. (Scores: 7 × 1 = 7)
Question 1.
What is the co-ordination number of particles present in FCC crystal structure?
Answer:
12
Question 2.
Identify the order of reaction if the unit of rate constant is mol L-1s-1.
Answer:
Zero order
Question 3.
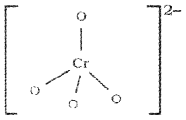
What is the structure of chromate ion ((CrO4)2-)?
Answer:
Tetrahedral
OR
Question 4.
Name the test used to identify primary amines using CHCl3 and ethanolic KOH.
Answer:
Carbylamine test/reaction or Isocyanide test
Question 5.
Which among the given vitamins is water soluble?
a) A
b) B
c) D
d) E
Answer:
b) B
Question 6.
What is the crosslinked polymer obtained by the polymerisation of phenol and formaldehyde?
Answer:
Bakelite
Question 7.
……….. is an artificial sweetener which is unstable at cooking temperature.
Answer:
Aspartame
(Questions 8 to 20): Answer any ten. Each question carries two scores. (Scores: 10 × 2 = 20)
Question 8.
a) Based on the nature of intermolecular forces, classify the following solids:
i) SiO2
ii) Ice
b) ZnO turns yellow on heating. Why?
Answer:
a) i) SiO2 – Covalent/Network solid
ii) Ice – Molecular slod/Hydrogen bonded molecular solid.
b) This is due to metal excess defect caused by the presence of extra cations at interstitial sites. ZnO is white in colour at room temperature. On heating it loses oxygen as,
![]()
The excess Zn2+ ions move to interstitial sites and electrons to neighbouring interstitial sites which imparts yellow colour to the crystal.
Question 9.
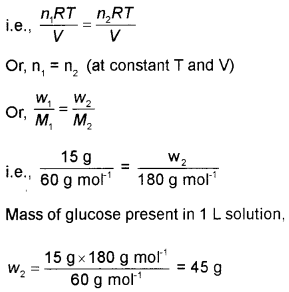
A solution contains 15g urea (molar mass = 60 g mol-1) per litre of solution in water has the same osmotic pressure as a solution of glucose (molar mass =180 g mol-1) in water. Calculate the mass of glucose present in one litre of its solution.
Answer:
πV = nRT
or π = \(\frac{n R T}{V}\)
Let π1 be the osmotic pressure of urea and π2 that of glucose. Then,
![]()
Here the two solutions are isotonic i.e., have same osmotic pressure.
Therefore, π1 = π2
Question 10.
Define minimum boiling azeotropes with example.
Answer:
Azeotropes are binary mixtures having the same composition in liquid and vapour phase and boil at a constant temperature. The solutions which show large positive deviation from Raoult’s law form minimum boiling azeotrope at a specific composition.
e.g. a 95% (v/v) solution of ethanol in water.
Question 11.
Write the chemical equation of the following reactions:
a) Preparation of XeO3 from XeF6.
b) Mixing PtF6 and Xe.
Answer:
a) XeF6 + 3H2O → XeO3 + 6HF
b) Xe + PtF6 → Xe + PtF6–
Question 12.
Explain how the complexes of nickel, [Ni(CN)4]2 and [Ni(CO)4] have different structures, but do not differ in their magnetic behaviour. (Ni, Atomic No: 28)
Answer:
In [Ni(CN)4]2-, Ni (3d8 4s2) is in +2 oxidation state (Ni2+ – 3d8) and it undergoes dsp2 hybridisation. So the ion has square planar geometry. But in [Ni(CO)4], Ni is in 0 oxidation state and it undergoes sp3 hybridisation. So it has tetrahedral geometry. Due to the absence of unpaired electrons both the complexes are diamagnetic.
Question 13.
Complete the reaction:
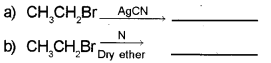
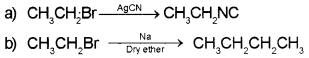
Answer:
Question 14.
During the β-elimination reaction of 2-bromopentane in an alcoholic solution of KOH results Pent-2-ene as major product and Pent-1-ene as minor product. State the rule to explain the reaction.
Answer:
Zaitsev rule/Saytzeff rule. The rule states that “in dehydrohalogenation reactions, if there is a possibility of formation of more than one alkene, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms.”
Question 15.
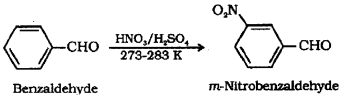
Aromatic aldehydes undergo electrophilic substitution reactions. Write the nitration reaction of benzaldehyde with chemical equation.
Answer:
The -CHO group being a meta-directing group, benzaldehyde on nitration gives m-nitrobenzaldehyde.
Question 16.
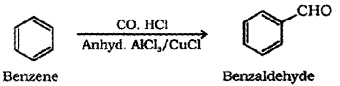
Briefly describe Gatterman Koch reaction.
Answer:
Gatterman – Koch reaction: When benzene or its derivative is treated with carbon monoxide and hydrogen chloride in the presence of anhydrous aluminium chloride or cuprous chloride, it gives benzaldehyde or substituted benzaldehyde.
Question 17.
How can it convert methyl iodide to ethanamine?
Answer:
Methyl iodide when treated with KCN or NaCN gives methyl cyanide or ethane nitrile which on reduction with LiAlH4 or H2 in presence of Ni/Pt/Pd or by sodium amalgam and ethanol we get ethanamine.
![]()
Question 18.
State two differences between globular and fibrous proteins.
Answer:
Globular proteins:
- Polypeptide chains coil around.
- Spherical shape.
- Soluble in water.
Fibrous proteins:
- Polypeptide chains run parallel and held together by hydrogen and disulphide bonds.
- Fibre-like structure.
- Insoluble in water.
Question 19.
Match the following:
| a) | Polyacrylonitrile | i) | Terylene |
| b) | 1,3-Butadien-Acrylonitrile | ii) | Natural Rubber |
| c) | Ethylene glycol-Terephthalic acid | iii) | Buna-N |
| d) | Cis-1, 4-polyisoprene | iv) | Acrilan |
Answer:
| a) Polyacrilonitrile | iv) Acrilan |
| b) 1,3-butadiene-Acrylonitrile | iii) Buna-N |
| c) Ethylene glycol-Terephthalic acid | i) Terylene |
| d) cis-1, 4-polyisoprene | ii) Natural Rubber |
Question 20.
a) What are drugs?
b) Write an example for a drug classified based on its chemical structure.
Answer:
a) Drugs are chemicals of low molecular mass (~ 100 – 500u) which interact with macro molecular targets and produce a biological response.
b) Sulphonamides or Sulpha drugs like arsphenamine/salvarsan, prontosil, sulphapyridine.
(Questions 21 to 29): Answer any seven. Each question carries three scores: (Scores: 7 × 3 = 21)
Question 21.
An element crystallises as FCC with density 2.8 gm3. Its unit cell having edge length 4 × 10-8 cm. Calculate the molar mass of the element. (Given NA = 6.022 × 1023 mol-1)
Answer:
d = 2.8 g cm-3, a = 4 × 10-8 cm, z = 4 (since unit cell is FCC), NA = 6.022 × 1023 mol-1.
Density, d = \(\frac{\mathrm{zM}}{\mathrm{a}^{3} \mathrm{~N}_{\mathrm{A}}}\)
Molar mass of the element, M = \(\frac{\mathrm{da}^{3} \mathrm{~N}_{\mathrm{A}}}{\mathrm{z}}\)
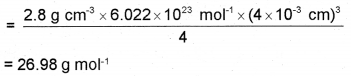
Question 22.
Write the anode and cathode reactions occur in the operation of a lead storage battery. Mention the electrolyte used in the battery.
Answer:
Anode reaction: Pb(s) + SO42- (aq) → PbSO4(s) + 2e–
Cathode reaction: PbO2(s) + SO42- (aq) + 4H+(aq) + 2e– → PbSO4(s) + 2H2O(l)
Electrolyte: 38% solution of sulphuric acid.
Question 23.
For hydrolysis of methyl acetate in aqueous solution, the following results were observed.
| t/s | 0 | 30 | 60 |
| CH3COOCH3 C/mol L-1 | 0.60 | 0.30 | 0.15 |
Show that it follows pseudo first order reaction as the concentration of water remains constant.
Answer:
As the concentration of water remains constant, practically the reaction should be pseudo first order with respect to methyl acetate.
The rate constant for a pseudo first order reaction,

From the above results it can be seen that k = k’ [H2O] is a constant and hence, it is a pseudo first order reaction.
Question 24.
a) State Hardy-Schulze rule with the help of example.
b) Why lyophilic colloids are used as protective colloids?
Answer:
a) The Hardy-Schulze rule states that the greater the valence of the flocculating ion added, the greater is its power to cause precipitation.
For example, in the coagulation of a negative sol like AS2S3, the flocculating power is in the order: Al3+ > Ba2+ > Na+
Or, in the coagulation of a positive sol like Fe2O3.xH2O, the flocculating power is in the order:
[Fe(CN)6]4- > PO43- > SO42- > Cl–
b) Lyophilic colloids have a unique property of protecting lyophobic colloids. When a lyophilic sol is added to the lyophobic sol, the lyophilic particles form a layer around lyophobic particles and thus protect the lyophobic sols from electrolysis.
Question 25.
Gibbs energy of formation (ΔfG) of MgO(s) and CO(g) at 1273 K and 2273 K are given below:
(ΔfG) [MgO(s)]: -941 kJ mol-1 at 1273 K

On the basis of the above data, predict the temperature at which carbon can be used as a reducing agent for MgO(s)
Answer:
As per the Ellingham diagram, for using carbon as a reducing agent for MgO(s), the value of ΔrG for the coupled reaction, MgO(s) + C(s) → Mg(s) + CO(g) should be negative at the required temperature.
Given that, at 1273 K
i) Mg(s) + \(\frac{1}{2}\)O2(g) → MgO(s); ΔfG = -941 kJ mol-1
ii) C(s) + \(\frac{1}{2}\)O2(g) → CO(g); ΔfG = -439 kJ mol-1
On reversing equation (i) and adding with equation (ii), we get
MgO(s) + C(s) → Mg(s) + CO(g); ΔrG = (941 + – 439) kJ mol-1 = +502 kJ mol-1
Since ΔrG is positive, carbon cannot be used as a reducing agent for MgO(s) at 1273 K.
Given that, at 2273 K
iii) Mg(s) + \(\frac{1}{2}\)O2(g) → MgO(s); ΔfG = -314 kJ mol-1
iv) C(s) + \(\frac{1}{2}\)O2(g) → CO(g); ΔfG = -628 kJ mol-1
On reversing equation (iii) and adding with equation (iv), we get
MgO(s) + C(s) → Mg(s) + CO(g); ΔrG = (314 + – 628) kJ mol-1 = -314 kJ mol-1
Since ΔrG is negative, carbon can be used as a reducing agent for MgO(s) at 2273 K.
Question 26.
a) What is the formula of the phosphine?
b) How phosphine is prepared in laboratory?
Answer:
a) PH3
b) In the laboratory, phosphine is prepared by heating white phosphorus with concentrated NaOH solution in an inert atmosphere of CO2.
P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2
(Or, any other laboratory method of preparation of PH3.)
Question 27.
Assign the possible reason for following:
a) Stability of +5 oxidation state decreases and that of +3 oxidation state increases down to 15th group elements.
b) H2O is less acidic than H2S.
c) H3PO2 act as a good reducing agent while H3PO4 does not.
Answer:
a) It is due to inert pair effect. In the case of heavier elements the lowest oxidation states are more prodominent due to non-participation of the s-electrons in the formation of bonds.
b) In the case of the hydrides (H2E) of group 16 elements, down the group the H-E bond dissociation enthalpy decreases and hence acidic character increases. Due to small size of O compared to that of S, the O-H bond dissociation enthalpy is greater than the S-H bond dissociation enthalpy. Hence, H2O is less acidic than H2S.
c) The reducing property of oxoacids of phosphorus depends on the P-H bond. The acids which contain P-H bond have strong reducing properties. Thus, H3PO2 is a good reducing agent as it contains two P-H bonds. H3PO4 has no P-H bonds and hence it has no reducing property.
Question 28.
Give reasons for the following:
a) Transition metals and many of their compounds act as catalyst.
b) Scandium (Z = 21) does not exhibit variable oxidation state and yet it is regarded as a transition element.
c) Write the step involved in the preparation of Na2CrO4 from chromite ore.
Answer:
a) Transition metals and their compounds are known for their catalytic activity. This is due to their ability to adopt multiple oxidation states and to form complexes. Thus they can form with lower activation energy for the reaction. Also because the transition metal ions can change their oxidation states, they become more effective as catalysts.
In some cases transition metals provide a suitable surface for the reaction to take place. The reactants are adsorbed on the surface of the catalyst where reaction occurs. This has the effect of increasing the concentration of the reactants at the catalyst surface and also weakening of the bonds in the reacting molecules.
b) Scandium is regarded as a transition element because it has incompletely filled 3d orbitals in the ground state of the atom (3d1 4s2).
c) The chromite ore is fused with sodium carbonate or potassium carbonate in free access of air.
4FeCr2O4 + 8Na2CO3 + 7O2 → 8Na2CrO4 + Fe2O3 + 8CO2
Question 29.
How would you account for the following:
a) Aldehydes are more reactive than ketones towards nucleophilic addition reaction.
b) Boiling point of aldehydes are lower than alcohols.
c) Addition reaction of sodium hydrogen sulphite is useful for separation and purification of aldehydes.
Answer:
a) This is due to steric and electronic reasons.
Sterically, the presence of two relatively large substituents in ketones hinders the approach of nucleophile to carbonyl carbon that in aldehydes having only one such substituent.
Electronically, the two alkyl groups reduce the electrophilicity of the carbonyl carbon more effectively in ketones than in aldehydes.
b) The boiling points of aldehydes are lower than those of alcohols of comparable molecular masses due to the absence of intermolecular hydrogen bonding in aldehydes where as alcohols can associate through intermolecular hydrogen bonding and have higher boiling points.
c) The hydrogen sulphite addition compound is water soluble and can be converted back to the original aldehyde by treating it with dilute mineral acid or alkali. Therefore, these are useful for separation and purification of aldehydes.
(Questions 30 to 33): Answer any three. Each question carries four scores: (Score: 3 × 4 = 12)
Question 30.
a) What are primary batteries?
b) The cell potential of a mercury cell is 1.35 V, and remain constant during its life. Give reason.
c) Write the equation of the reactions involved at each electrode in a H2-O2 fuel cell.
Answer:
a) Batteries in which the reaction occurs only once and after use over a period of time they become dead and cannot be reused again.
b) This is because the overall reaction does not involve any ion in solution whose concentration can change during its life time.
c) Anode reaction: 2H2(g) + 4OH– (aq) → 4H2O(l) + 4e–
Cathode reaction: O2(g) + 2H2O(l) + 4e– → 4OH– (aq)
Question 31.
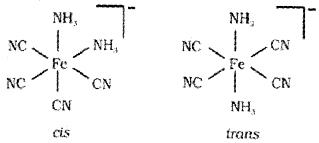
a) Draw the structures of geometrical isomers of [Fe(NH3)2(CN)4]–
b) Write the formula of pentaammine carbonate cobalt (III) chloride.
c) Wife any two limitations of valance bond theory.
Answer:
a)
b) [Co(NH3)5(CO3)]Cl
c) i) It involves a number of assumptions.
ii) It does not give quantitative interpretation of magnetic data.
iii) It does not explain the colour exhibited by coordination compounds.
iv) It does not give a quantitative interpretation of the thermodynamic or kinetic stabilities of coordination compounds.
v) It does not make exact predictions regarding the tetrahedral and square planar structures of 4-coordinate complexes
vi) It does not distinguish between weak and strong ligands.
(Any two limitations)
Question 32.
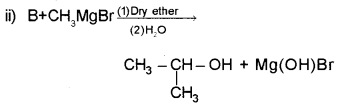
a) Grignard reagents are important class of organometallic compounds used to prepare alcohols. Identify the compounds A and B and write the formula.
![]()
b) Write the name of products formed when salicylic acid is treated with acetic anhydride in acid medium
Answer:
a) i) Compound A : Ethanol/Ethyl alcohol – CH3CH2OH
ii) Compound B : Ethanal/Acetaldehyde – CH3CHO
b) Acetylsalicylic acid/Aspirin and Acetic acid/Ethanoic acid
Question 33.
Lucas test is used to identify primary, Secondary and tertiary alcohols.
a) Explain the process.
b) Name the reagents used in the test.
Answer:
a) Lucas test – When treated with Lucas reagent, tertiary alcohols give turbidity immediately, secondary alcohols give turbidity within 5 minutes while primary alcohols do not give turbidity at room temperature but only on heating. This test is based upon the relative reactivities of various alcohols towards Lucas reagent. The order of reactivity is, 3° alcohols > 2° alcohols > 1° alcohols. Alcohols are soluble in Lucas reagent and form a clear solution. On reaction, alkyl chlorides are formed which being insoluble result in the development of turbidity,
b) Lucas reagent is a mixture of conc. HCl and anhydrous ZnCl2.
