Kerala Plus Two Chemistry Previous Year Question Paper Say 2018 with Answers
| Board | SCERT |
| Class | Plus Two |
| Subject | Chemistry |
| Category | Plus Two Previous Year Question Papers |
Time: 2 Hours
Cool off time: 15 Minutes
Maximum: 60 Score
General Instructions to candidates:
- There is a ‘cool off time’ of 15 minutes in addition to the writing time of 2 hrs.
- Use the ‘cool off time’ to get familiar with the questions and to plan your answers.
- Read questions carefully before you answering.
- Read the instructions carefully.
- Calculations, figures, and graphs should be shown in the answer sheet itself.
- Malayalam version of the questions is also provided.
- Give equations wherever necessary.
- Electronic devices except non-programmable calculators are not allowed in the Examination Hall.
Answer all questions from 1 to 7. Each question carries 1 score. (7 × 1 = 7)
Question 1.
If N spheres are there in a close packing, what is the total number of tetrahedral and octahedral voids present in it?
Answer:
Tetrahedral 2N, octahedral N
Question 2.
What is the order of a reaction, if its half life is independent of initial concentration?
Answer:
1st order
Question 3.
What is the magnetic moment of an atom having d configuration.
Answer:
Zero
Question 4.
Gabriel synthesis of used forthe preparation of which type of amines?
i) Primary
ii) Secondary
iii) Tertiary
iv) Quaternary
Answer:
i) Primary
Question 5.
Which vitamin is responsible for blood clotting?
Answer:
Vitamin K
Question 6.
Name the linear polymer formed during the condensation polymerization between phenol and formaldehyde.
Answer:
bakelite
Question 7.
Which is the chemical substance discovered by Paul Ehlrich for the treatment of syphilis?
Answer:
Salvarsan or Arephenamine
II. Questions from 8 to 20 carry 2 score each. Answer any 10 questions. (10 × 2 = 20)
Question 8.
Draw the vapour pressure-mole fraction curve for a non-ideal solution having positive deviation, if A and B are the two volatile components.
Answer:
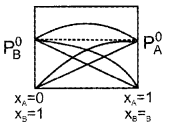
Question 9.
Calculate the depression in freezing point of a 0.2 molal solution if kg for water is 1.86 K kg mol-1.
Answer:
ΔTf = kjm
= 1.86 × 0.2
= 0.372K
Question 10.
Suppose you are given a sample of NaCl salt. How will you prepare chlorine gas in laboratory using the above sample? (Write balanced chemical equations)
Answer:
By the electrolysis of sodium chloride solution Cl2 gas can be prepared
2NaCl + 2H2O → 2Na+ + 2OH– + H2+(g) + Cl2(g)
Question 11.
Give one use each of Freon 12, DDT, CCl4and CHl3.
Answer:
Freon – 12-Refrigerant
DDT – insecticide
CCl4 – For the manufacture of refrigerant
CHl3 – Antiseptic
Question 12.
Write equations showing Wurtz-Fittig reaction and Fittig reaction.
Answer:
Fitting reaction
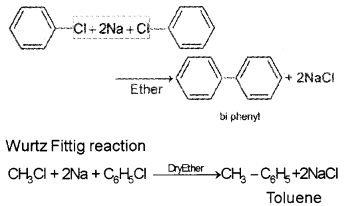
Question 13.
Identify A and B in the following equations:
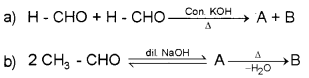
Answer:
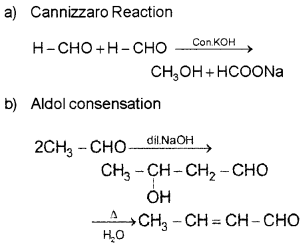
Question 14.
How the conversion of carbon dioxide to carboxylic acid can be effected using. Grignard reagent?
Answer:
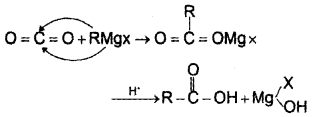
Question 15.
Complete the following equations:
![]()
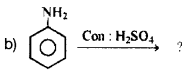
Answer:
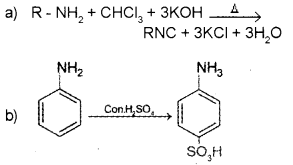
Question 16.
Describe primary and secondary structure of proteins.
Answer:
Structure of proteins:
1) Primary structures – amino acids are arranged in sequence.
2) Secondary structure:
a) α – helix – polypeptide chains are coild to form a helical structure eg. Myosine
b) β-pleated structure: amino acid chains lie side by side and bonded by hydrogen bonds, eg. Keratine.
Question 17.
Explain homopolymers and copolymers with examples.
Answer:
Polymers formed by polymerisation of one type of monomer are called homopolymer, eg. Polythene Polymers formed by polymerisation 0 two or more different monomers are called copolymers eg. SBR, Rubber, Nylon 6, 6.
Question 18.
Briefly explain different types of artificial sweetening agents.
Answer:
Commonly used artificial sweetener is saccharin. It is 550 times sweeter than sucrose. Alitame is 1000 times sweet as cane sugar.
Aspartame, monolellin etc are other sweetening agent.
Question 19.
Write the IUPAC names of the following compounds:
a) [Ni(CO)4]
b) K3[Fe(C2O4)3]
Answer:
a) Ni(CO)4 Tetra carbonyl nickel (0)
b) K3 [Fe(C2O4)3] Potassium tris oxalate ferrate iii
Question 20.
Distinguish Ferromagnetism and Ferrimagnetism.
Answer:
Ferromagnetic substance – Magnetic moments are in one direction.
![]()
They are strongly attracted my magnetic field, eg Fe, Co
Ferrimagneticsubstances: Magnetic moments are unequal and in opposite direction.

eg. Fe3O4, MgFe2O4
III. Questions from 21 to 29 carry 3 score each. Answer any 7 questions. (7 × 3 = 21)
Question 21.
Silver atoms are arranged in CCP lattice structure. The edge length of its unit cell is 408 pm. Calculate the density of silver. (Atomic mass of silver is 108.4)
Answer:
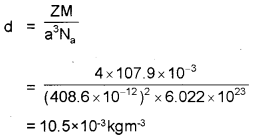
Question 22.
The rate of a reaction quadruples when the temperature changes from 293 K to 313 K. Calculate the energy of activation of the reaction assuming that it does not change with temperature.
Answer:
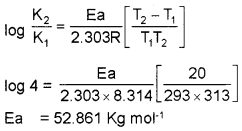
Question 23.
Explain any three chemical methods for the preparation of Lyophobic colloids with suitable examples.
Answer:
1) Oxidation methods: Oxidation of aqueous solution of H2S with SO2
SO2 + 2H2S → 3S + 2H2O
2) Reduction method : Reduction of AuCl3 solution using SnCl2
2AuCl3 + 3SNCl2 → 3SnCl4 + 2Au
3) Hydrolysis. By adding a saturated solution of ferric .chloride dropwise to a large excess of boiling water
FeCl3 + 3H2O → Fe(OH)3 + 3HCl
Question 24.
Explain the following refining processes:
a) Distillation
b) Vapour phase refining
c) Zone refining
Answer:
a) Distillation: The impure metal is heated to form pure metals as distillate it is collected and condensed impurities are left behind, eg Zn and Hg.
Only metals with low boiling point can apply this method.
b) Vapour phase refining
i) Van Arkel method – eg Titanium, Zirconium, Thorium etc.
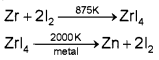
Mond process: Nickel is strongly heated with carbon monoxide to form Nickel tetra carbonyl this is again heated strongly to get pure nickel.

c) The impure metal bar is heated at one end with moving circular heater. The heater is now slowly moved along the rod. The pure metal recrystallises from the melt while impurities remain in the melt. Finally the end where impurities have collected is cut off. The impure metal bar is heated at one end with moving circular heater. The heater is now slowly moved along the rod. The pure metal recrystallises from the melt while impurities remain in the melt. Finally the end where impurities have collected is cut off.
Question 25.
A solution of CuSO4 is electrolysed for 20 minutes with a current of 1.5 amperes. What is the mass of copper deposited at cathode?
(Atomic mass of copper – 63)
Answer:
Cu2+ + 2e → Cu
Q = It 1.5 × 20 × 60 = 1800 C
Mass of Cu deposited by 1800 C
\(\frac{63.5 \times 1800}{2 \times 96500}\) = 0.5875 g
Question 26.
Briefly explain the manufacture of sulphuric acid by contact process.
Answer:
Contact Process
1) Sulphur is burnt in air to form Sulphur Dioxide
S + O2 → SO2
2) Sulphur Dioxide is again oxidised to SO3 with atmospheric oxygen in the presence of
![]()
3) SO3 is treated with Sulphuric acid to get Oleum
SO3 + H2SO4 → H2S2O7
4) Oleum is diluted to get H2SO4
H2S2O7 + H2O → 2H2SO4
Question 27.
Explain with the help of equations, preparation of Xenon fluorides.
Answer:
Xe + F2 → XeF2
Xe + 2F2 → XeF4
Xe + 3F2 → XeF6
Question 28.
Describe lanthanoid contraction. Write any two consequences of it.
Answer:
The steady but slow decrease in the size of atoms or ions of the lanthanoids with increase in atomic number is called Lanthanoid Contraction.
Consequences:
- As the size of the Lanthanoid ions decreases from La to Lu. The covalent character of hydroxides increases and hence the basic strength decreases.
- The change in ionic radii of lanthanoids is very small their properties are almost similar. This makes the separation of lanthanoids are very difficult.
Question 29.
How the conversion of an aldehyde to acetal can carried out?
(Write chemical equations)
Answer:
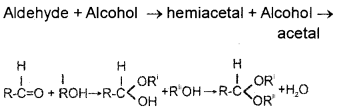
IV. Questions from 30 to 33 carry 4 score each. Answer any 3. (3 × 4 = 12)
Question 30.
Predict the products of electrolysis of the following substances at anode and cathode using suitable chemical equations.
a) Aqueous NaCl
b) H2SO4 solution
Answer:
Electrolysis of aqeous NaCl
a) At Cathode H+ + 1e– → \(\frac{1}{2}\)H2
As the standard reduction potential for H+ ions are more it is easily reduced at cathode.
At anode Ch ions are oxidised Cl– → Cl + e
2Cl → Cl2(g)
b) Electrolysis of Sulphuric acid (dilute)
At anode
2H2O → O2 + 4H+ + 4e–
At cathode
H+ + 1e → H
H + H → H2
Electrolysis of concentrated H2SO4
At cathode
H+ + 1e → H
H + H → H2
At anode
2SO\(\mathrm{O}_{4}^{2-}\) → S2O\(\mathrm{O}_{8}^{2-}\) + 2e
Question 31.
Draw a diagram depicting crystal field splitting in an octahedral environment of d-orbitals. Label the diagram properly. Calculate the crystal field stabilization energy for a d3 configuration.
Answer:
Crystal field splitting in octahedral field.
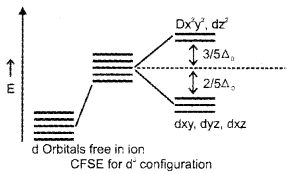
CFSE for d3 configuration in octa hedral field. CFSE for 3 unpaired electrones
0 – \(\frac{2}{5}\)Δ0 × 3 = \(\frac{-6}{5}\)Δ0
Question 32.
a) Predict the products A and B.
![]()
b) How methanol is prepared industrially?
Answer:
2CH3 – CH = CH2 + (BH3)2 → (CH3 – CH2 – CH2)3 B → CH3 – CH2 – CH2 – OH
By the catalytic hydrogenation of carbon monoxide in presence of a catalyst at 573K and under 200 to 300 atmospheric pressure to form methanol
![]()
Question 33.
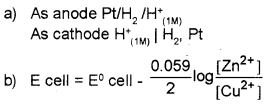
a) Symbolically represent standard hydrogen electrode, when it acts as an anode and as cathode.
b) Write Nernst equation for a Daniel cell.
(Assume activity of metals is unity)
Answer: