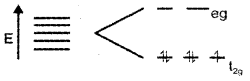Kerala Plus Two Chemistry Previous Year Question Paper March 2019 with Answers
| Board | SCERT |
| Class | Plus Two |
| Subject | Chemistry |
| Category | Plus Two Previous Year Question Papers |
Time: 2 Hours
Cool off time: 15 Minutes
Maximum: 60 Score
General Instructions to candidates:
- There is a ‘cool off time’ of 15 minutes in addition to the writing time of 2 hrs.
- Use the ‘cool off time’ to get familiar with the questions and to plan your answers.
- Read questions carefully before you answering.
- Read the instructions carefully.
- Calculations, figures, and graphs should be shown in the answer sheet itself.
- Malayalam version of the questions is also provided.
- Give equations wherever necessary.
- Electronic devices except non-programmable calculators are not allowed in the Examination Hall.
I. Answer all questions from 1 to 7. Each carries 1 score. (7 × 1 = 7)
Question 1.
The monomeric unit of natural rubber is …………
Answer:
Isoprene or 2-methyl 1, 3 butadiene or
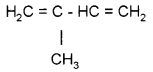
Question 2.
The weakest reducing agent among the hydrides of group 15 elements is …………
Answer:
Ammonia or NH3.
Question 3.
The reaction in which an amide is converted into a primary amine by the action of Br2 and alcoholic NaOH is known as ………….
Answer:
Hoffmann bromamide reaction.
Question 4.
\(\mathrm{MnO}_{4}^{-}\) and ……… are formed by the disproportionation of \(\mathrm{MnO}_{4}^{2-}\) in acidic medium.
Answer:
MnO2 or Mn4+.
Question 5.
In a solution of components‘A’ and ‘B’, at molecular level, A – B interactions are weaker than those between A – A or B – B interactions. Then the type of deviation shown by this solution is called ……………
Answer:
Positive deviation.
Question 6.
Identify the co-ordination compound which can exhibit linkage isomerism, among the following.
a) [Pt(NH3)2Cl2)
b) [Co(NH3)5(SO4)]Br
c) [CO(NH3)5(NO2)]Cl2
d) [Cr(NH3)6][CoF6]
Answer:
c) [CO(NH3)5(NO2)]Cl2
Question 7.
For the reaction, 2NO(g) + O2(g) → 2NO2(g), the rate law is given as,
Rate = k[NO]2 [O2], The order of the reaction with respect to O2 is …………
Answer:
one
II. Answer any ten questions from 8 to 20. Each carries 2 scores. (10 × 2 = 20)
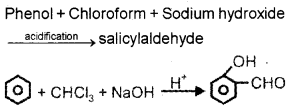
Question 8.
Write the chemical equation representing Reimer-Tiemann reaction.
Answer:
Question 9.
What is reverse osmosis? Write any one of its applications.
Answer:
If the pressure applied is larger than osmotic pressure the direction of osmosis gets reversed. It is called reverse osmosis.
Aplication:
- Desalination of sea water.
- Purification of drinking water.
Question 10.
Identify the products and give the name of the following reaction:
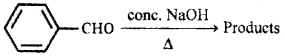
Answer:
Cannizzaro reaction:
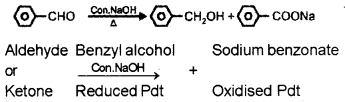
Question 11.
Explain Haloform reaction.
Answer:
Compounds with CH3 – CO or CH3 – CH – OH gp only gives haloforms such compounds when react with sodium hypohalite or a mixture of halogen and NaOH gives haloform.
![]()
Question 12.
What is meant by step growth polymerisation? Give an example.
Answer:
Step growth polymers are condensation polymers. The eliminate simple molecules like water or ammonia on addition, eg. nylon 6,6
Question 13.
An element crystallizes in F.C.C. manner. What is the length of a side of the unit cell, if the atomic radius of the element is 0.144 nm?
Answer:
a = 2\(\sqrt{2}\)r
r = 0.144 nm
= 2\(\sqrt{2}\) × 0.144 = 0.407 nm
Question 14.
Draw the structure of H3PO2 and account for its reducing character.
Answer:
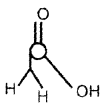
Due to the presence of two P – H bonds it is a strong reducing agent.
Question 15.
2-Bromobutane is optically active. Explain the stereo¬chemical aspect of SN1 reaction of 2-Bromobutane with OH- ions.
Answer:
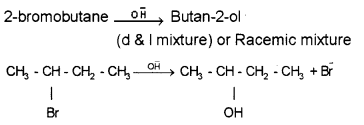
Question 16.
Briefly explain the different types of emulsions and give examples for each.
Answer:
Emulsion: Both dispersed phase & dispersion medium are liquids:
1) Oil in water type, e.g. milk, vanishing cream.
2) Water in oil type, butter.
Question 17.
Give the structural formula and IUPAC name of the product formed by the reaction of propanone with CH3MgBr in dry ether, followed by hydrolysis.
Answer:
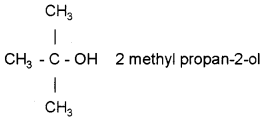
Question 18.
Examine the graph given below. Identify the integrated rate equation and the order of the reaction corresponding to it.
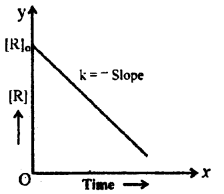
Answer:
Order – zero
Rate equation for zero order K = \(\frac{[\mathrm{Ro}]-[\mathrm{R}]}{\mathrm{t}}\)
Question 19.
How is primary amine distinguished from a secondary amine using a chemical test?
Answer:
A) Carbyl amine test: Only primary amine gives carbyl amine test. Secondary amine does not give this test, (foul smelling gas).
(OR)
B) Hinsberg test: Primary amine react with benzene sulphonyl chloride to form a precipitate which is soluble in alkali. But the precipitate formed by the secondary ammine is insoluble in alkali.
(OR)
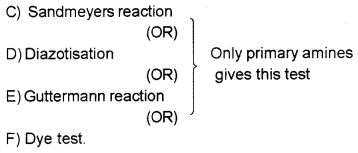
Question 20.
Predict the products obtained by the reaction of 2-methoxy-2-methyl propane with HI.
Answer:
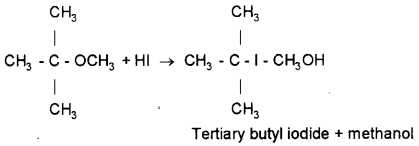
III. Answer any seven questions from 21 to 29. Each carries 3 scores. (7 × 3 = 21)
Question 21.
Explain the terms, Zeta potential, electrophoresis and electro-osmosis.
Answer:
Zeta potential: The potential difference between the fixed layer and the diffused layer of an electrical, double layer.
Electrophoresis: The movement of colloidal particles under the influence of an electric field.
Electro osmosis: The movement of particles of dispersion median under the influence of electric field.
Question 22.
The rate constant of a reaction at 293 K is 1.7 × 105 s-1. When the temperature is increased by 20K, the rate constant is increased to 2.57 × 106 s-1. Calculate Ea and A of the reaction.
Answer:
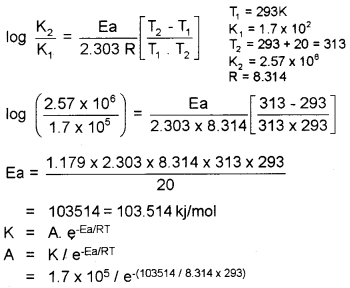
Question 23.
Identify A, B and C in the following sequence of reactions:
![]()
Answer:
A = CH3CONH2
B = CH3-COOH
C = CH2Br – COOH
Question 24.
Briefly explain different types of neurologically active drugs and give exmaple for each type.
Answer:
Tranquilizers: Medicines or chemicals used for mental-stress, e.g. Equanil, barbituric acid, veronal, luminal, seconal (anyone).
Analgesics are painkillers and are also neurologically active drug, e.g. paracetamol, morphine, heroine.
Question 25.
Write any three applications of d-block and f-block elements.
Answer:
- They act as catalyst.
- They form alloys.
- Cu, Ag Au are coinage metals.
- They have different oxidation states.
Question 26.
Give the open chain and ring structures of glucose and account for the existence of glucose in two anomeric forms.
Answer:
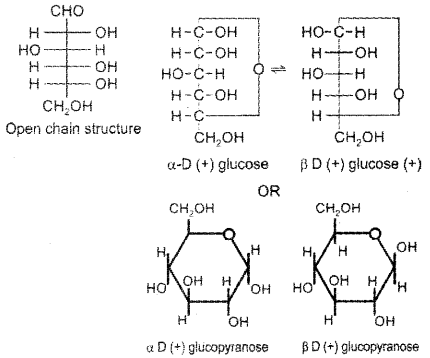
Question 27.
A 5% solution (by mass) of cane sugar (C12H22O11) in water has a freezing point of 271 K. Calculate the freezing point of 5% (by mass) solution of glucose (C6H12O6) in water. Freezing point of pure water is 273.15 K.
Answer:
ΔTf = \(\frac{1000 \mathrm{~K}_{\mathrm{f}} \cdot \mathrm{W}_{2}}{\mathrm{~W}_{1} \cdot \mathrm{M}_{2}}\)
for 5% suger solution
W2 = 5
W1 = 100 – 5 = 95
M2 = 342
Tf = 271 k
T°f = 273.15
ΔTt= T°f – Tf = 2.15 K
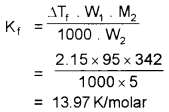
For 5% gulcose solution W2 = 5, W1 = 100 – 5 = 95g, M2 = 180
Question 28.
Explain the steps involved in the vapour phase refining of Ni and Zr.
Answer:
Ni by Monds process.
Ni is strongly heated at a temperature of 330 – 350K to form nickel tetra carbonyl. It is again heated strongly 450 – 470 to decompose it.
![]()
Zr by Van Arkel method.
Zirconium is treated with iodine to form zirconium tetra iodide. It is electrically heated to about 1800K with tungsten filament. Zr I4 is decomposed
Zr + Zl2 → Zrl4
Zrl4 → Zr + 2l2
Question 29.
What are inter halogen compounds? Which interhalogen compound is used to fluorinate Uranium? How is it prepared?
Answer:
Inter halogen compounds are formed by the direct combination of halogen.
![]()
CIF3 or Br F3 are used to Fluorinate Uranium.
They are compounds of two or more halogen atoms.
IV. Answer any three questions from 30 to 33. Each carries 4 scores. (3 × 4 = 12)
Question 30.
How can the following conversions be effected?
i) Ethanol-Fluroethane
ii) But-l-ene → But-2-ene
Answer:
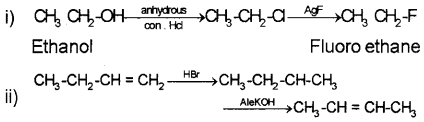
Question 31.
Diagrammatically represent H2 – O2 fuel cell and write the half cell reactions taking place in this cell.
Answer:
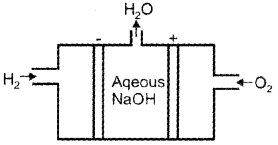
Reaction at cathode
O2g + 2H2(l)O + 4\(\bar{e}\) → 4O\(\bar{H}\)(ag)
Reaction at anode
2H2(g) + 4O\(\bar{H}\)(aq) → 4H2O(I)+ 4\(\bar{e}\)
2H2 + O2 → 2H2O
Question 32.
What are point defects? Explain the non-stoichiometric point defects in ionic crystals.
Answer:
Point defect: The irregularities from ideal arrangement around a point or an atom in crystalline substance.
Non-stoichiometric defect: The defect which do not disturb the stoichiometry of the crystalline substance.
I) Metal excess defect:
- Due to anion vacancies. Electron trapped anion vacancies are called F centre which gives colour.
- Due to extra cation at interstitial site.
II) Metal deficiency defect:
- Due to cation vacancies.
Question 33.
i) With the help of a diagram, give the splitting of d-orbitals of Mn2+ ion and octahedral crystal field.
ii) On the basis of crystal field theory, explain why [Mn(H2O)6]2+ contains five unpaired electrons while [Mn(CN)6]4- contains only one unpaired electron.
Answer:
i) Splitting of d orbital in octahedral system.
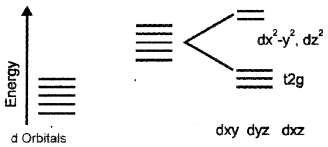
ii) In [Mn (H2O)6]2+ Mn2+ have five electrons.
As (H2O) is a weak ligand, i.e., Δ < P no pairing occurs and electronic configuration t2g3 eg2.
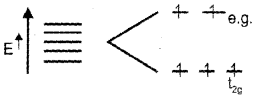
But in [Mn(CN)6]4- Mn2+ has five electrons and are in paired state as CN– is a strong ligend and Δ > P pairing occurs the electronic configuration is t2g5 eg0.