Plus Two Chemistry Notes Chapter 9 Coordination Compounds is part of Plus Two Chemistry Notes. Here we have given Plus Two Chemistry Notes Chapter 9 Coordination Compounds.
| Board | SCERT, Kerala |
| Text Book | NCERT Based |
| Class | Plus Two |
| Subject | Chemistry Notes |
| Chapter | Chapter 9 |
| Chapter Name | Coordination Compounds |
| Category | Plus Two Kerala |
Kerala Plus Two Chemistry Notes Chapter 9 Coordination Compounds
Coordination chemistry – branch of chemistry which deal with the complex compounds formed by tmasition and other metals. Chlorophyll, haemoglobin and vitamin B12 are coordination compounds of Mg, Fe and Co respectively.
Werner’s Theory of Coordination Compounds
The main postulates are,
- Metal posses two types of valencies-primary and secondary. The primary valency is ionisable while the secondary valency is non-ionisable.
- Every metal atom or ion has a fixed number of secondary valancies equal to its coordination number.
- The primary valencies are satisfied by negative ions and the secondary valencies by negative or neutral groups (ligand).
- The ligand satisfying the secondary valencies are always directed towards fixed positions in space giving a definite geometry to the complex. The primary valencies are non directional.
Some Important Terms in Coordination Compounds
(a) Coordination Entity, a central metal atom or ion bonded to fixed number of ions or molecules.
e.g. [Ni(CO)4], [Fe(CN)6]-4.
(b) Central Atom/Ion: the cation to which one or more neutral molecules or anions are attached, e.g. In [Fe(CN)6]-4, Fe2+ is central ion.
(c) Ligand: ions or molecules bound to the central atom/ ion in the coordination entity.
1. Unidentate/monodentate ligand: provides one electron pair per molecule, e.g. NH3, H2O, CO, F–, Cl– etc.
2. Bidentate/didentate ligand: furnishes two lone pair of electron per molecule, e.g. ethane -1,2- diamine or ethylenediamine (en) NH2 – CH2 – CH2 – NH2 Oxalate ion (ox) C2O42-.
3. Polydentate ligand: provides several pairs of electrons i.e. they e.g. EDTA (ethylene diamine tetraacetate) is hexadentate ligand.
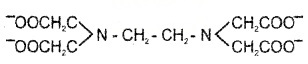
4. Chelate: bidentate or polydentate ligand which binds to a single central metal atom/ion and forms a ring like structure.
5. Ambidentate Ligand: Ligand which can ligate through two different atoms.
e.g. -NO2 & -ONO, -SCN & -NCS.
(d) Coordination number (C.N): number of ligand donor atoms to which the metal is directly bonded. e.g. [Ni(CO)4] C.N = 4 [CO(en)3] C.N = 6
[PtCl6]2- C.N = 6.
(e) Coordination Sphere: The central atom along with ligands surrounding it, written in a square bracket. The atoms, ions or molecules in this sphere are non-ionisable. The ionisible groups are written outside the bracket and are called counter ions.
e.g. K4[Fe(CN)6]: [Fe(CN)6]4- – Coordination sphere, K+ -Counterion.
(f) Coordination Polyhedron: The spatial arrangement of the ligand atoms which are directly attached to the central atom/ion. e,g. octahedral, square planar and tetrahedral.
(g) Oxidation Number of Central Atom: The charge that the central atom in a complex would carry if all the ligands are removed along with electron pairs. It is represented by Roman numeral in parenthesis, e.g. [Co(NH3)6]3+, O.N of Co is +3 i.e., Co(III).
(h) Homoleptic Complexes: Complexes in which a metal is bound to only one kind of donor groups (ligands). e.g. [Co(NH3)6]3+.
Heteroleptic Complexes: Complexes in which a metal is bound to more than one kind of donor groups (ligands), e.g. [Co(NH3)4]Cl2]+.
Nomenclature of Coordination Compounds
1. The positive part of the coordination compound is named first and is followed by the name of negative part.
2. The ligands are named first followed by the central metal. The ligands are named in alphabetical order.
3. The prefixes di, tri, tetra etc. are used to indicate the number of same kind of ligands present. The prefixes bis(two ligands), tris (three ligands) etc. are used when the ligand include numerical prefixes, e.g. Ethylenediamine, dipyridyl.
4. Names of the anionic ligands ends in -’o’, those of cationic in ‘ium’. Neutral ligands have their regular names H2O- aqua, NH3– ammine, NO – nitrosyl, CO – carbonyl.
5. The O.N. of the central metal is indicated in Roman numeral in parenthesis.
6. When a complex species has negative charge, the name of the metal ends in ‘ate’, e.g. [Co(SCN)4]2-Tetrathiocyanatocobaltate(II).
For some metals, the Latin names are used in the complex anions, e.g. Ferrate for Fe, Argentate for Ag. If the complex ion is a cation, the metal is named same as the element.
Some examples:
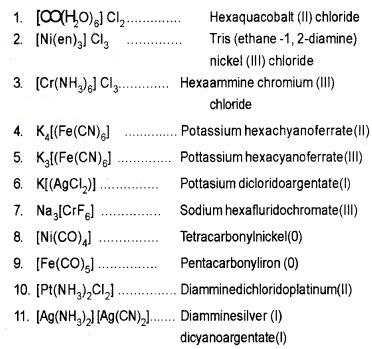
Isomerism in Coordination Compounds
Isomers – two or more compounds that have same chemical formula but a different arrangement of atoms. Coordination compounds exhibit structural and stereo isomerism.
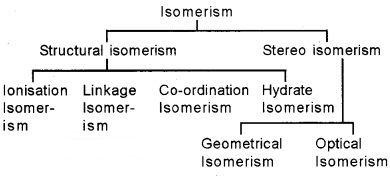
1. Structural Isomerism:
(i) Ionisation Isomerism: arises when the counter ion in a complex salt is itself a potential ligand and can displace a ligand which can then become the counter ion.
e.g. [Co(NH3)5Br] SO4 – (Violet) gives [Co(NH3)5Br]2+ + SO42-
[CO(NH3)5SO4] Br – (Red) gives [Co(NH3)5SO4]+ + Br–
(ii) Linkage Isomerism: arises in a coordination compound containing ambidentate ligand, e.g.
[CO(NH3)5NO2]Cl2 – Pentamminenitrito-N-cobalt(III) chloride.
[CO(NH3)5ONO]Cl2 – Pentamminenitrito-N-cobalt(III) chloride.
(iii) Coordination Isomerism: arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex, e.g. [Cr(NH3)6] [Co(CN)6] & Co(NH3)6] [Cr(CN)6].
(iv) Hydrate Isomerism or Solvate Isomerism-, these isomers differ by whether or not a solvent molecule (water) is directly bonded to the metal ion or merely present as free solvent molecules in the crystal lattice.
e.g. [Co(H2O)6]Cl3(Violet)
[CO(H2O)5Cl] Cl2.H2O (Blue Green)
[Co(H2O)4Cl2]Cl.2H2O (Green)
2. Stereo Isomerism:
Exhibited by compounds containing same ligand and central metal ion, but different spacial arrangement of ligands.
1. Geometrical Isomerism: arises in heteroleptic complexes due to different possible geometric arrangements of the ligands. Geometrical isomerism in square planar complexes: e.g. [Pt(NH3)2Cl2]
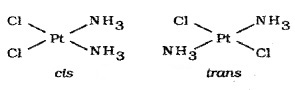
It can occur with any square planar complexes of the type [ M X2L2] or [ML2XY]. It cannot occur in tetrahedral complexes because all positions in a tetrahedral complex are equivalent.
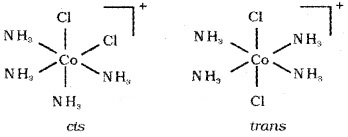
Geometrical isomerism in octahedral complexes: Octahedral complexes of the type [ M X2L4] or [ M XYL4] exist as cis and trans isomers.
e.g. [Co(NH3)4Cl2]+
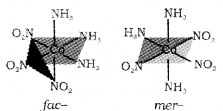
fac- mer isomerism – occurs in octahedral complex of the type [MX3Y3]. If the three donor atoms of the same ligands occupy adjacent positions at the corners of an octahedral face, it is facial (fac) isomer. When the positions are around the meridian of the octahedron, it is merdional (mer) isomer, e.g. [CO(NH3)3(NO2)3]
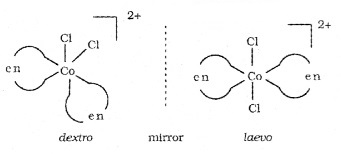
Optical Isomerism:
Ability of a compound to rotate the plane polarised light. Dextro (right) rotatory – compound which can rotate plane polarised light towards right.
Laevo rotatory – compound which can rotate plane polarised light towards left. Optical isomerism common in octahedral complexes involving didentate ligands.
Bonding in coordination compounds
1. Valence Bond Theory (VBT) (By Pauling): the central metal atom/ion can use (n-1)d, ns, np or ns, np, nd orbitals for hybridisation to yield a set of equivalent orbitals of definite geometry which are allowed to overlap with ligand orbitals that can donate electron pair for bonding.
- C.N 4 – sp3 hybridisation – tetrahedral
- C.N 4 – dsp2 hybridisation – square planar
- C.N 5 – sp3d hybridisation – trigonal bipyramidal
- C.N 6 – sp3d2 hybridisation – octahedral
- C.N 6 – d2sp3 hybridisation – octahedral
e.g. (i) [Co[NH3)6]3+ – cobalt ion is in +3 oxidation state and has electronic configuration 3d6. In presence of NH3 ligand the 3d electrons are paired and two d – orbitals, one s orbital and three p orbital undergo d2sp3 hybridisation.
Since the innerd-orbital (3d) is used in hybridisation it is called an inner orbital or low spin or spin paired complex. All electrons are paired, hence the molecule is diamagnetic.
(ii) [CoF6]3- is octahedral, paramagnetic (4 unpaired electrons), the outer d-orbital(4d) is used in the hybridisation (sp3d2). Thus it is called outer orbital or high spin or spin free complex.
(iii) [NiCl4]2- – Ni is in the +2 oxidation state (3d8), sp3 hybridisation, tetrahedral, paramagnetic (2 unpaired electrons).
(iv) [Ni(CO)4] – Ni is in 0 oxidation state, sp3 hybridisation, tetrahdral. diamagnetic (no unpaired electron).
(v) [Ni(CN)4]2- – Ni is in +2 oxidation state (3d8), dsp2 hybridisation, square planar, diamagnetic (no unpaired en.).
2. Magnetic Properties of Coordination Compounds:
The structures adopted by metal complexes can be explained by measuring their magnetic moments. For 3d1, 3d2 and 3d3 configurations there are two vacant d orbitals for hybridisation with 4s and 4p orbitals. The magnetic behaviour of these free ions and their coordination entities is similar.
For 3d4, 3d5, 3d6 etc. Configurations the required pair of 3d orbitals for octahedral hybridisation results only by pairing of 3d electrons which leaves unpaired electrons. The magneticdata agree with maximum spin pairing in many cases (Complications in d4 and d5 ions).
e.g.
- [Mn(CN)6]3- – (Mn3+ – 3d4) – paramagnetic- 2 unpaired electrons.
- [MnCl6]3- – (Mn3+ – 3d4) – paramagnetic – 4 unpaired electrons.
- [Fe(CN)6]3- – (Fe3+ – 3d5) – paramagnetic-1 unpaired electron.
- [FeF6]3- – (Fe3+ – 3d5) – paramagnetic – 5 unpaired electrons.
- [CoF6]3- – (Co3+ – 3d6) – paramagnetic – 4 unpaired electrons.
- [Co(C2O4)3]3- – (Co3+ – 3d6) – diamagnetic.
This can be explained in terms of formation of inner orbital and outer orbital coordination entities.
[Mn(CN)6]3-, [Fe(CN)6]3- and [Co(C2O4)3]3- – inner orbital complexes – d2sp3 hybridisation.
[MnCl6]3-, [FeF6]3- and [CoF6]3- – outer orbital complexes – sp3d2 hybridisation.
3. Limitations of VB theory:
- Involves a number of assumptions.
- Does not give quantitative interpretation of magnetic data.
- Does not explain the colour of coordination compounds.
- Does not give a quantitative interpretation of the thermodynamic or kinetic stabilities of coordination compounds.
- Does not make exact predictions regarding the tetrahedral and square planar structures of 4- coordinate complexes.
- Does not distingish between weak and strong ligands.
4. Crystal Field Theory (CFT): It considers the metal-ligand bond to be ionic arising purely from electrostatic interactions between the metal ion and the ligand. Ligands are treated as a point charges in the case of anions or dipoles in case of neutral molecules.
The degeneracy of d-orbitals is removed when negative field is due to ligands. This results in splitting of the d-orbitals, the pattern of which depends upon the nature of the crystal field.
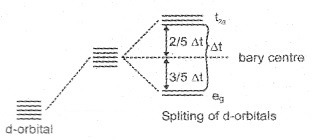
a. Crystal Field Splitting in Octahedral Complexes:
Here the metal atom is surrounded by six ligands. The orbital lying along the axes i.e., dz2 & d2x−y2 experience more repulsion and will be raised in energy; and the dxy, dyz and dxz orbitals will be lowered in energy from the average energy in the spherical crystal field.
Thus the degeneracy of the d-orbitals is removed to yield three orbitals of lower energy (t2g set) and two orbitals of higher energy (eg set). This splitting of the degenerate orbital due to the presence of ligands in a definite geometry is termed as crystal field splitting.
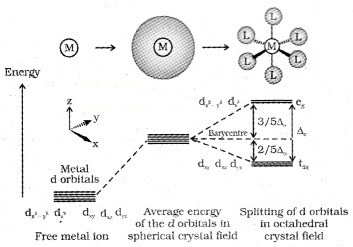
The crystal field splitting (∆0) depends upon the filed produced by the ligand and charge on the metal ion.
Spectrochemical Series: The series in which ligands are arranged according to their increasing field strength. The order is as given below:
l– < Br– < SCN– < Cl– < S2- < F– < OH–C2O42- < H2O < NCS– < edta4- < NH3 < en < CN– < CO
Electronic configuration in t2g and eg orbitals.

Ligands for which ∆0 < P are known as weak field ligands and form high spin complexes. Ligands for which ∆0 > P are known as strong field ligands and form low spin complexes.
b. Crystal Field Splitting in Tetrahedral Compounds:
Here the d-orbital splitting is inverted and is smaller as compared to octahedral splitting. ∆t = (4/9) ∆0
5. Colour in Coordination Compounds:
The colour of a transition metal complex is complementary to that which absorbed. It can be explained in terms of CFT. e.g. [Ti(H2O)6]3+. In Ti3+ (3d1) the single electron is present in the t2g level (t2g1).
When white light passes through the solution it absorb yellow-green light which would excite the electron to eg level (t2g1 eg0 → t2g0 eg1) and the complex appears violet in colour (d-d transition).
Bonding in Metal Carbonyls
The homoleptic carbonyls are formed by most of the transition metals.
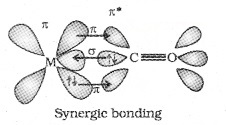
e.g. [Ni(CO)4], [Fe(CO)5], [Cr(CO)6], [Mn(CO)5]. The metal-carbon bond in metal carbonyls posses both ‘s’ and ‘p’ character. The M-C σ bond is formed by the donation of lone pair of electrons on the carbonyl carbon into a vacant orbital of the metal.
The M-C π bond is formed by the donation of a pair of electrones from a filled d-orbital of metal into the vacant antibonding π* orbital of CO. The metal to ligand bonding creates a synergic effect which strengthens the bond between CO and the metal.
Stability of Coordination Compound
The stability of a complex in solution refers to degree of association between the two species involved in the state of equilibrium. Higherthe stability constant (or formation constant) higher the stability of the compound.
Importance and Applications of Coordination Compounds
- In qualitative and quantitative chemical analysis.
- Eestimattion of hardness of H2O (titration with EDTA).
- Extraction of some metals, like Ag and Au.
- Purification of nickel (Mond process).
- In biological systems, e.g. Chlorophyll, vitamin B12 etc.
- As catalysts for many industrial process, e.g. Wilkinson catalyst – [(Ph3P)3 RhCI] – for the hydrogenation of alkenes.
- In black and white photography.
- In medicine – Some coordination compounds of Pt effectively inhibit the growth of tumours, e.g. cis-platin. EDTA is used in the treatment of lead poisoning.
We hope the Plus Two Chemistry Notes Chapter 9 Coordination Compounds help you. If you have any query regarding Plus Two Chemistry Notes Chapter 9 Coordination Compounds, drop a comment below and we will get back to you at the earliest.
