Plus Two Chemistry Notes Chapter 15 Polymers is part of Plus Two Chemistry Notes. Here we have given Plus Two Chemistry Notes Chapter 15 Polymers.
| Board | SCERT, Kerala |
| Text Book | NCERT Based |
| Class | Plus Two |
| Subject | Chemistry Notes |
| Chapter | Chapter 15 |
| Chapter Name | Polymers |
| Category | Plus Two Kerala |
Kerala Plus Two Chemistry Notes Chapter 15 Polymers
The word polymer is coined from two Greek words poly means many and mer means unit. They are also called macromolecules.
Monomers – the repeating units of a polymer derived from some simple and reactive molecules. Polymerisation – process of formation of polymers from respective monomers.
e.g. nCH2 = CH2 (ethene) → -(CH2 – CH2)-n (polyethene).
Classification of Polymers
1. Based on Source:
- Natural Polymers: naturally occuring polymers, found in plants and animals, e.g. cellulose, rubber,
wool, starch. - Semi-synthetic Polymers: ploymers obtained by modifying the naturally occuring polymers.
e.g. cellulose nitrate, cellulose acetate (rayon) - Synthetic Polymer: polymers synthesised by chemical processes, e.g. nylon 6,6, Buna – S, etc.
2. Based on the Structure of Polymers:
- Linear Polymers: polymers consisting of long and straight chains, e.g. polythene, polyvinyl chloride (PVC), etc.
- Branched Chain Polymers: polymers containing linear chains having some branches, e.g. low density polythene (LDPE).
- Cross Linked Polymers: polymers formed from bi-functional and tri-functional monomers. They contain strong covalent bonds between various linear chains, e.g. bakelite, melamine, etc.
3. Based on mode of polymerisation:
(1) Addition Polymers:
Polymers formed by the repeated addition of monomers possessing double or tripple bonds, e.g. polyethene, PVC, polystyrene, etc.
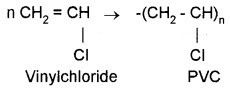
Homopolymers:
Addition polymers formed by the polymerisation of a single monomeric species.
e.g. polythene, polystyrene, etc.
Copolymers:
Polymers made by addition polymerisation of two different monomers.
e.g. Buna-S, Buna-N, etc.
(2) Condensation polymers:
Polymers formed by repeated condensation reaction between two different bi-functional ortri-functional monomeric units, e.g. nylon – 6,6.
4. Based on Molecular Forces:
(1) Elastomers:
Rubber-like solids with elastic properties, polymer chains are held together by the weakest intermolecular forces which stretching. The ‘cross links’ introduced help to retract the polymer to its original position after the force is released.
e.g. buna – S, buna – N, neoprene, etc.
(2) Fibres:
Thread forming solids which possess high tensile strength and high modulus due to strong intermolecular forces like H-bonding.
e.g. nylon -6,6, polyesters(terylene), etc.
(3) Thermoplastic Polymers:
Linear or slightly branched long chain molecules capable of repeatedly softening on heating and hardnening on cooling. The inter molecular forces of attraction are intermediate between elastomers and fibres.
e.g. polyethene, polystryne, polyvinyls, etc.
(4) Thermosetting polymers:
Cross linked or heavily branched molecules, which on heating undergo extensive cross linking in moulds and again become infusible. These cannot be reused.
e.g. bakelite, urea-formaldehyde resins, etc.
Types of Polymerisation Reactions
a. Addition Polymerisation or Chain Growth Polymerisation:
Same or different monomers (unsaturated compounds) add together on a large scale through the formation of either free radicals or ionic species.
1. Free radical mechanism-It is characterised by 3 steps.
- Initiation – a free radical is generated in presence of organic peroxide catalyst.
- Propagation – The bigger radicals formed carries the reaction forward.
- Termination – The product radical reacts with another radical to form the polymerised product. e.g. polymerisation of ethene to form polyethene in presence of benzoyl peroxide.
2. Preparation of Some Important Addition Polymers
(a) Polythene:
There are two types of polythene.
(i) Low-Density Polythene (LDP):
Obtained by the polymerization of ethene under high pressure of 1000 to 2000 atm and temperature 350 to 570 K, has a highly branched structure.
Uses: In the insulation of electrical wires; manufacture of squeeze bottles, toys and flexible pipes.
(ii) High-Density Polythene (HDP):
Formed when ethene is polymerized in a hydrocarbon solvent in presence of Ziegler-Natta catalyst (Triethylaluminium and TiCl4 at 333 K – 343 K and 6 – 7 atm. It has high density due to close packing, chemicaly inert, more tougher and harder. Uses: for making buckets, dust bins, bottles, pipes, etc.
(b) Polytetrafuoroethene (Teflon):
Formed by the polymerisation of tetrafluoroethene in presence of free radical or persulphate catalyst at high pressure.
Uses: for the preparation of oil seals, gaskets, nonstick surface coated utensils.

(c) Polyacrylonitrile (PAN):
Formed by the addition polymerisation of acrylonitrile in presence of peroxide catalyst.

Uses: as a substitue for wool in making commercial fibres as orlon or acrilan.
b. Condensation Polymerisation or Step Growth Polymerisation:
It involves repetitive condensation reaction between two bi-functional monomers.
(1) Polyamides:
Polymers possessing amide (-CO-NH-) linkages.
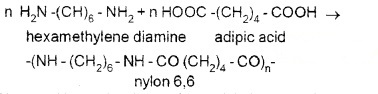
(i) Nylon 6, 6:
Prepared by the condensation of hexamethylenediamine and adipic acid. Uses:in making sheets, bristles for brushes and in textile industry.
(ii) Nylon 6:
Obtained by heating caprolactam with water at a high temperature.
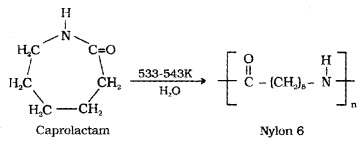
Uses: manufacture of tyre cords, fabrics and ropes.
(2) Polysters:
Polycondensation products of dicarboxylic acids and diols.
(i) Dacron or Teriyne:
Manufactured by heating a mixture of ethylene glycol and terephthalic acid at 420 to 460 K in the presence of zinc acetate-antimony trioxide catalyst.
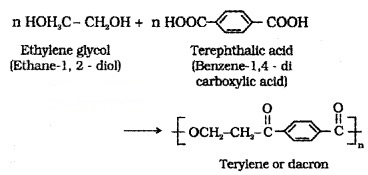
Uses: in blending with cotton and wool fibres, as glass reinforcing materials in safety helmets.
(3) Phenol-Formaldehyde Polymer:
Phenol and formaldehyde undergo condensation reaction in the presence of either an acid or a base catalyst. The initial linear product formed is called Novolac. It is used in paints.
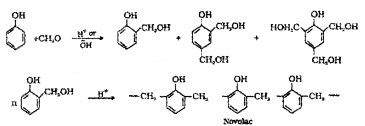
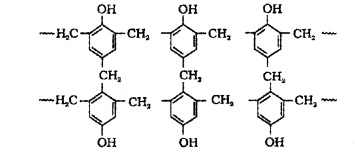
Novalac on heating with formaldehyde undergoes cross linking to form bakelite. Uses: for making combs, phonograph records, electrical switches and handles of various utensils.
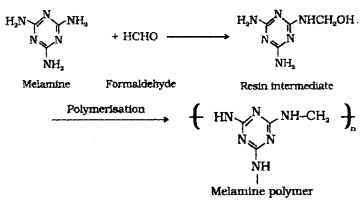
(4) Melamine – Formaldehyde Polymer:
Formed by the condensation polymerisation of melamine and formaldehyde. Use: for making unbreakable crockery.
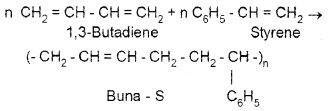
c. Copolymerisation:
Polymerisation reaction in which a mixture of more than one monomeric species is allowed to polymerise and form copolymer, e.g. Buna – S. It is quite tough and is a good substitute for natural rubber. Uses: for the manufacture of automobile tyres, floortiles, foorwear components, cable insulation, etc.
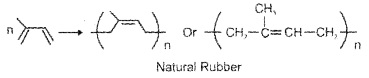
d. Natural Rubber:
Natural polymer, possess elastic properties, obtained from rubber latex, polymer of isoprene (2 – methyl-1, 3-butadiene), also called cis-1, 4-polyisoprene.
(1) Vulcanisation of Rubber:
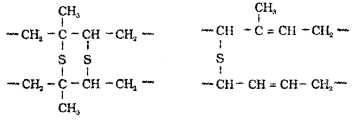
Process of heating natural rubber with sulphur at about 373 K to 415 K To improve its physical properties. On vulcanisation, sulphur forms cross links at the reactive sites of double bonds and thus the rubber gets stiffened.
(2) Synthetic Rubber:
Any vulcanisable rubber-like polymer.
(i) Neoprene (polychloropren): Formed by the free radical polymerisation of chloroprene.
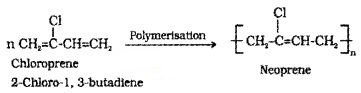
It has supreior resistance to vegetable oils and mineral oils. Uses: for manufacturing conveyor belts, gaskets and hoses.
(ii) Buna – N:
Prepared by the copolymerisation of 1,3 – butadiene and acrylonitrile in the presence of a peroxide catalyst.
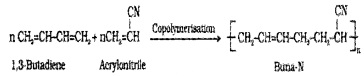
It is resistanttothe action of petrol, lubricating oil and oiganic solvents. Uses: in making oil seals, tank lining, etc.
Biodegradable Polymers
Polymers which can overcome environmental problems caused by polymeric solid waste materials, can be broken into small fragments by enzyme catalysed reaction, e.g.
(1) Poly β -hydroxybutyrate – co – β- hydroxy valerate (PHBV):
Obtained by the copolymerisation of 3 – hydroxybutanoic acid and 3-hydroxypentanoic acid. Uses: in speciality packaging, orthopaedic devices, in controlled release of drugs.
(2) Nylon -2, Nylon -6:
Alternating polyamide copolymer of glycine and aminocaproic acid, biodegradable.
H2N – CH2 – COOH + H2N – (CH2)5 – COOH → -(NH – CH2 – CO – NH – (CH2)5 – CO )-n
Polymers of Commercial Importance
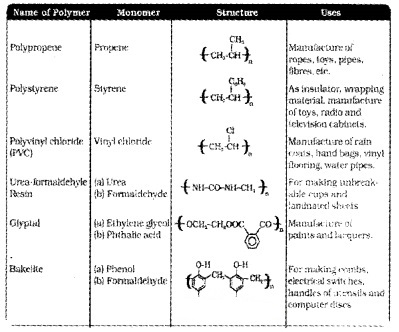
We hope the Plus Two Chemistry Notes Chapter 15 Polymers help you. If you have any query regarding Plus Two Chemistry Notes Chapter 15 Polymers, drop a comment below and we will get back to you at the earliest.
