Plus Two Chemistry Notes Chapter 12 Aldehydes, Ketones and Carboxylic Acids is part of Plus Two Chemistry Notes. Here we have given Plus Two Chemistry Notes Chapter 12 Aldehydes, Ketones and Carboxylic Acids.
| Board | SCERT, Kerala |
| Text Book | NCERT Based |
| Class | Plus Two |
| Subject | Chemistry Notes |
| Chapter | Chapter 12 |
| Chapter Name | Aldehydes, Ketones and Carboxylic Acids |
| Category | Plus Two Kerala |
Kerala Plus Two Chemistry Notes Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Carbonyl compounds – compounds containing the carbonyl group.
![]()
- Aldehydes – compounds in which the carbonyl group is bonded to a carbon and hydrogen.
- Ketones – compounds in which the carbonyl group is bonded to two carbon atoms.
Carboxylic acids and their derivatives (esters, anhydrides) – compounds in which the carbonyl group is bonded to oxygen.
- Amides – compounds in which the carbonyl group is bonded to nitrogen atom.
- Acyl halides – compounds in which the carbonyl group is bonded to halogen atom.
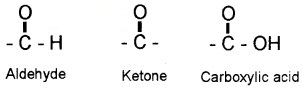
Nomenclature and Structure of Carboxyl Group
(1) Aldehydes and ketones:
(a) Common names:
Derived from the common names of the corresponding carboxylic acids by replacing ‘ic’ of the acids with ‘aldehyde’.
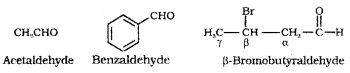
IUPAC names:
IUPAC names of aliphatic aldehydes or ketones are derived from the names of the corresponding alkanes. The ending ‘e’ of the alkane is replaced by ‘al’ for aldehyde and ‘one’ for ketone.
CH3 – CHO (Ethanal)
CH3 – CO – CH3 (Propanone)
Structure of the Carbonyl Group:
The carbonyl carbon atom is sp2 – hybridised. It forms 3 sigma bonds and one π bond. The carbonyl group has a trigonal coplanar structure.
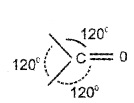
The ![]() bond is polarised due higher electronegativity of oxygen relative to carbon.
bond is polarised due higher electronegativity of oxygen relative to carbon.
Preparation of Aldehydes and Ketones
1. By Oxidation of Alcohols:
1° alcohols on oxidation give aldehydes and 2° alcohols on oxidation gives ketones.
2. By Dehydrogenation of Alcohols:
When the vapours of 1° alcohols are passed over heated Cu catalyst at 573 K corresponding aldehydes are formed while 2° alcohols on similar treatement give corresponding ketones.
3. From Hydrocarbons:
(i) Ozonolysis of alkenes:
It involves the addition of ozone molecule to alkene to form ozonide, and then cleavage of the ozonide by Zn – H20 to aldehydes, ketones or both.
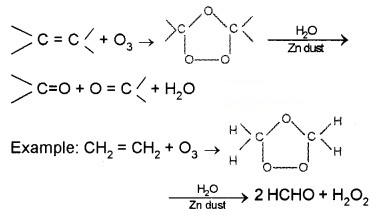
a. Preparation of Aldehydes:
(1) Rosenmund reduction – From acid chloride:
Acid chloride is hydrogenated over catalyst, Pd on BaSO4 to form aldehyde.
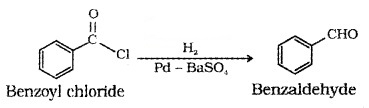
(2) From Nitriles and Esters (Stephen reaction):
Nitriles are reduced to immine with SnCl2/HCl which on hydrolysis gives aldehydes.
![]()
Nitriles are selectively reduced by DIBAL – H (Diisobutylaluminium hydride) to imines followed by hydrolysis to aldehydes.
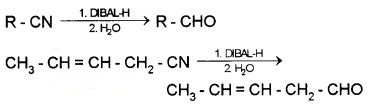
Esters are reduced to aldehydes with DIBAL -H.
![]()
(3) From Hydrocarbons:
Aromatic aldehydes are prepared from aromatic hydrocarbons.
(i) By Oxidation of Methyl Benzene:
(a) Etard Reaction – Toluene or substituted toluene when treated with chromyl chloride (CrO2Cl2) the methyl group is oxidised to a chromium complex, which on hydrolysis gives corresponding benzaldehyde.
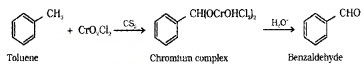
(b) Using Chromic Oxide (CrO3):
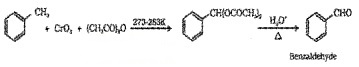
(ii) By side chain chbrination followed by hydrolysis:
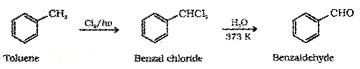
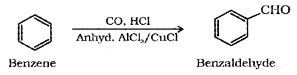
(iii) Gatterman – Koch Reaction:
When benzene or its derivative is treated with CO and HCl in the presence of anhydrous AlCl3 or CUCl, it gives benzaldehyde or substituted benzaldehyde.
b. Preparation of Ketones
(1) From Acyl Chlorides:
Treatment of acyl chlorides with dialkylcadmium gives ketones.
2 R – Mg – X + CdCl2 → R2Cd + 2Mg(X)Cl
2 R’ – CO – Cl + R2Cd → 2 R’ – CO – R + CdCl2
(2) From Nitriles:
Nitriles on treating with Grignard reagent followed by hydrolysis yield ketones.
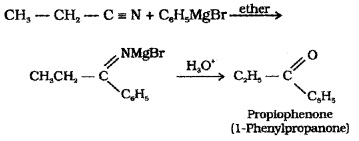
(3) From Benzene or Substituted Benzenes (Friedel – Crafts acylation):
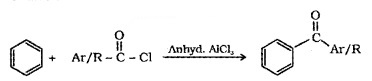
Physical Properties
The boiling points of aldehydes and ketones are higher than those of hydrocarbons and ethers (due to dipole-dipole interaction) but lower than those of alcohols (due to absence of intermolecular hydrogen bonding).
Lower members are miscible with water (due to formation of hydrogen bond). Their solubility decreases rapidly on increasing the length of alkyl chain.
Chemical Reactions
(1) Nucleophilic Addition Reactions:
(i) Mechanism:
A nucleophile attacks the carbonyl C. Its hybridisation changes from sp2 to sp3 and a tetrahedral alkoxide intermediate is formed which captures a proton to form the product.
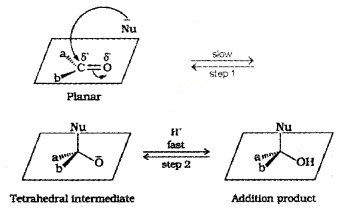
(ii) Reactivity: Aldyhydes are generally more reactive than ketones in nucleophilic addition reaction due to:
Steric reason – presence of relatively large substituents in ketones hinders the approach of nucleophile to carbonyl carbon than in aldehydes having only one such substituent.
Electronic reason – two alkyl groups reduce the electrophilicity of the carbonyl carbon more effectively in ketones than in aldehydes.
(iii) Some important examples of nucleophilic addition and nucleophilic addition-elimination reactions:
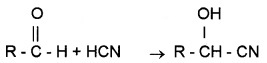
(a) Addition of HCN:
Cyanohydrins are formed.
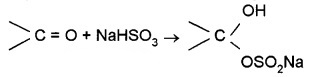
(b) Addition of Sodium Hydrogensulphite:
Corresponding crystalline addition products are formed which are water-soluble and can be converted back to the original carbonyl compound by treating with dilute mineral acid or alkali. Therefore, these are useful for separation and purification of aldehydes.
(c) Addition of Grignard Reagent:
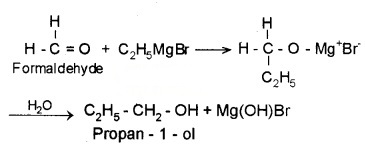
Addition products are formed which on hydrolysis give alcohols by the reaction of Grignard reagents with aldehydes and ketones. The adduct formed by the nucleophilic addition of RMgX to carbonyl group on hydrolysis yeilds alcohol.
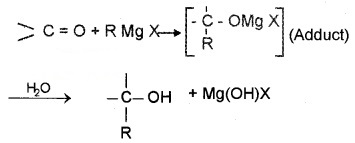
- Formaldehyde (HCHO) gives 1° alcohols
- Other aldehydes (R – CHO) give 2° alcohols
- Ketones (R-CO-R) give 3° alcohols
Example:
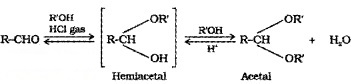
(d) Addition of Alcohols:
Aldehydes react with one equivalent of alcohol in presence of dry HCI to form hemiacetal, which further react with alcohol to form acetals.
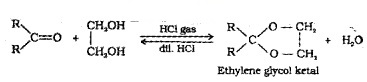
Ketones react with ethylene glycol in presence of dry HCI to form cyclic products known as ethylene glycol ketals.
(e) Addition of Ammonia and Its Derivatives:
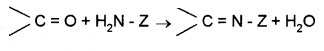
Z = alkyl, aryl, -OH, -NH2, C6H5NH–, -NHCONH2 etc.
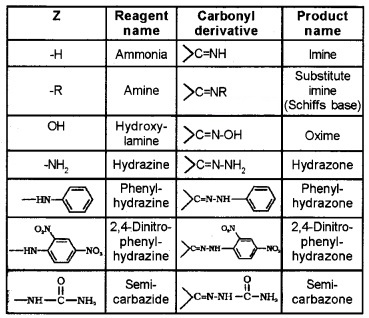
(2) Reduction:
(i) Reduction to Alcohols:
Aldehydes are reduced to 1° alcohols while ketones are reduced to 2° alcohols by NaBH4, LiAlH4 or by catalytic hydrogenation these are reduced using LiAlH4, NaBH4, H2/Pd, etc. Aldehyde gives 1° alcohols, while ketones gives 2° alcohols.
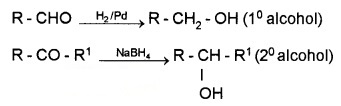
By the Reduction of Carboxylic Acids or Esters:
(ii) Reduction to Hydrocarbons:
(a) Clemmensen reduction:
The carbonyl group of aldehydes and ketones are reduced to – CH2 – group on treatment with zinc amalgam and concentrated HCl.
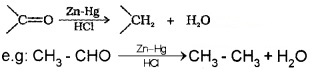
(b) Wolff – Kishner reduction:
The carbonyl group of aldehydes and ketones are reduced to – CH2 – group on treatment with hydrazine followed by heating with KOH in high boiling solvent such as ethylene glycol.
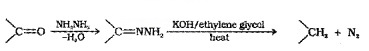
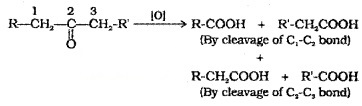
(3) Oxidation:
Oxidation of aldehyde gives carboxylic acids with same number of carbon atoms. The common oxidising agents used are HNO3, KMnO4, K2Cr2O7. Even mild oxidising agents like Tollens’ reagent and Fehling’s reagent also oxidise aldehydes.
![]()
Ketones are oxidised under vigorous conditions to give mixture of carboxylic acids having lesser number of C atoms than the parent ketone.
(i) Tollens’ Test (Tollen’s Reagent – Ammonical AgNO3):
On warming an aldehyde with Tollens’ reagent a bright silver mirror is produced due to the formation of silver metal. Aldehydes are oxidised to corresponding carboxylate ion. Ketones do not respond to this test.
R – CHO + 2 [Ag(NH3)2]+ + 3 OH– → R – COO– + 2 Ag + 2H2O + 4NH3
(ii) Fehling’s Test:
Fehling reagent is mixture of two solutions:|
Fehling solution A-aq. CuSO4 & Fehling solution B-Alkaline sodium potassium tartarate (Rochelle salt). On heating an aldehyde with Fehling’s reagent, a reddish-brown ppt. of Cu2O is obtained. Aromatic aldehydes & ketones do not respond to this test.
R – CHO + 2 Cu2+ + 5 OH– → R – COO– + Cu2O + 3H2O.
(iii) Oxidation of Methyl Ketones by Haloform Reaction:
Aldehydes and ketones having at least one CH3– group linked to carbonyl C are oxidised by sodium hypohalite to corresponding carboxylic acids having one C less than that of carbonyl compound.
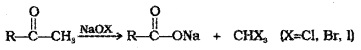
Iodoform reaction with NaOl is also used for detection of CH3CO- group or CH3-CH(OH)- group which produce CH3-CO- group on oxidation.
(4) Reactions Due to α-Halogen:
(i) Aldol Condensation:
Aldehydes and ketones having at least one α-hydrogen react in presence of dilute alkali to form β-hydroxy adehydes (aldol) or β-hydroxy ketones (ketol) respectively.
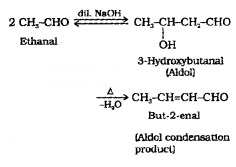
(5) Other Reactions:
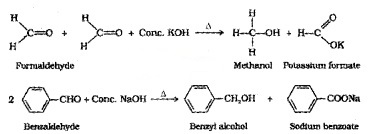
(i) Cannizzaro Reaction:
Aldehydes which do not have an α-H atom, undergo self oxidation-reduction (disproportionation) reaction on treatment with cone, alkali. In this reaction one molecule of the aldehyde
is reduced to alcohol while another is oxidised to carboxylic acid salt.
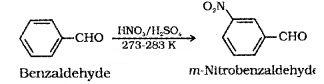
(ii) Electrophilic Substitution:
Here the carbonyl group acts as a deactivating and meta directing group.
Uses of Aldehydes and Ketones:
As solvent in industry; 40% HCHO solution is formalin; HCHO is used to prepare bakeite, urea-formalde glues and other polymeric products; C6H5CHO is used in perfumery and in dye industries; Butyraldehyde, vanaline, acetophenone, camphor are well known for their odours and flavours; Acetone and ethyl methyl ketones are common industrial solvents.
Carboxylic Acids
Compounds containing – COOH group. R – COOH – Aliphatic acid. Ar- COOH -Aromatic acid.
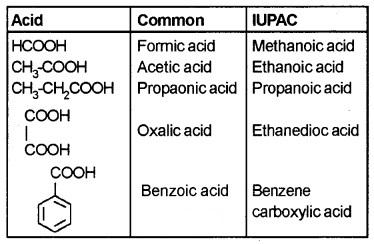
Nomenclature:
Common names – derived from Latin and Greek names.
IUPAC names – ‘e’ of alkane is replanced by ‘-oic’ acid.
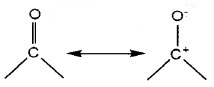
Structure of Carboxylic Group:
The bonds to the carboxyl C lie in one plane and are separated by about 120°. The carboxylic carbon is less electrophilic than carbonyl carbon because of resonance.
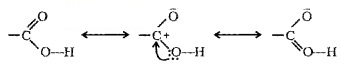
Methods of Preparation of Carboxylic Acids
(1) From primary alcohols and aldehydes:
10 alcohols are readily oxidised to carboxylic acids using KMnO4 in neutral, acidic or alkaline media or by K2Cr2O7 and CrO3 in acidic media.
![]()
Carboxylic acids can be prepared from aldehydes even using mild oxidising agents.
![]()
(2) From alkylbenzenes:
On vigorous oxidiation using chromic acid or acidic or alkaline KMnO4, the entire side chain of the alkyl benzene is oxidised to carboxyl group irrespective of length of the side chain.
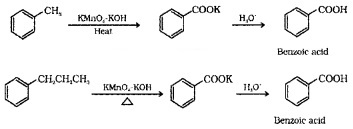
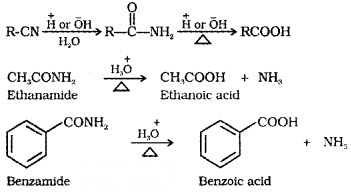
(3) From Nitriles and Amides:
Nitriles are hydrolysed to amides and and then to carboxylic acid in the presence of H+ or OH– as catalyst.
(4) From Grignard Reagent:
Grignard reagents react with CO2 (dry ice) to form salts of carboxylic acids which on acidification with mineral acids give corresponding carboxylic acids.
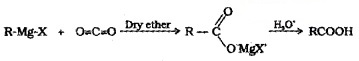
(5) From Acid Chlorides and Acid Anhydrides:
Acid chlorides when hydrolysed with water give carboxylic acids. Acid chlorides are hydrolysed with aqueous base to give carboxylate ions which on acidification gives corresponding carboxylic acids.
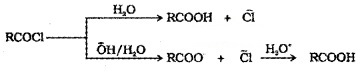
Anhydrides are hydrolysed to corresponding acids with water.
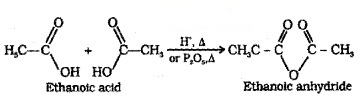
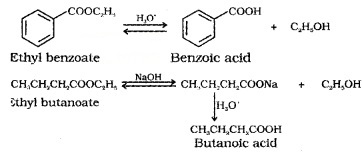
(6) From Esters:
Acidic hydrolysis of esters gives carboxylic acids while basic hydrolysis gives carboxylates, which on acidification give corresponding carboxylic adds.
Physical Properties
They have high boiling points than aldehydes, ketones, and even alcohols of comparable molecular mass due to more extensive association through intermolecular hydrogen bonding.
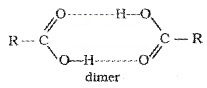
Chemical Reactions
a. Reactions Involving Cleavage of O-H Bond:
Acidity – Reaction with metals and alkalies:
Carboxylic acids evolve hydrogen with electropositive metals and form salts with alkalies.
2 R – COOH + 2 Na → 2R – COO–Na+ + H2
R – COOH + NaOH → R – COO– Na+ + H2O
R – COOH + NaOH → R – COO–Na+ + H2O + CO2
Carboxylic acids are Bronsted acids. The acidity is explained by the resonance stabilization of the carboxylate ion.
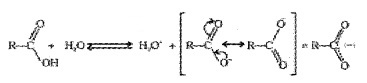
Carboxylate ion is more resonance stabilized than phenoxide ion. Hence, carboxylic acids are more acidic than phenol.
Effect of Substituents on the Acidity of Carboxylic Adds:
Electron withdrawing groups increase the acidity of carboxylic acids by stabilizing the conjugative base through delocalization of the negative charge by inductive and/or resonance effects. But electron-donating groups decreases the acidity by destabilizing the conjugate base.
The effect of groups in increasing acidity is in the order: Ph < I < Br < Cl < F < CN < NO2 < CF3
The presence of an electron-withdrawing group on the phenyl group of aromatic carboxylic acids increases their acidity while electron-donating groups decrease their acidity.
b. Reactions Involving Cleavage of C-OH Bond:
(1) Formation of Anhydride:
Carboxylic acids on heating with mineral acids such as H2SO4 or with P2O5 give anhydride.
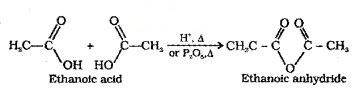
(2) Esterification:
Carboxylic acid are esterified with alcohols or phenols in presence of mineral acids.
![]()
(3) Reaction with PCl5, PCl3, and SOCl2:
R – COOH + PCl5 → R – COCl + POCl3 + HCl
3 R – COOH + PCl3 → 3 R – COCl + H3PO3
R – COOH + SOCl2 → R – COCl + SO2 + HCl
Thionyl chloride (SOCl2) is preferred because the other two products (SO2 and HCl) are gaseous and escape the reaction mixture making the pruification of the products easier.
(4) Reaction with Ammonia:
Ammonium salts are formed which on further heating give amides.
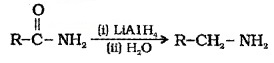
c. Reactions Involving -COOH Group:
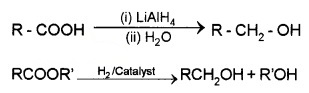
(1) Reduction:
Carboxylic acids are reduced to primary alcohols by LiALH4 or better with B2H6.
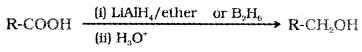
(2) Decarboxylation:
Carboxylic acids lose CO2 and form hydrocarbon when theirsodium salts are heated with sodalime (NaOH + CaO).
![]()
Kolbe’s electrolysis:
![]()
4. Substitution Reactions in the Hydrocarbon Part:
(1) Halogenation – Hell-Volhard-Zelinsky (HVZ) Reaction:
Carboxylic acids having an α – hydrogen are halogenated, at the α – position on treatment with Cl2 or Br2 in presence of small amount of red P to give α – halo carboxylic acids.

(2) Ring substitution:
Aromatic carboxylic acids undergo electrophilic substitution reactions in which the -COOH group acts as deactivating and meta-directing group. They donot undergo Friedel – Crafts reaction because the carboxyl group is deactivating and the catalyst AlCl3 gets bonded to the carboxyl group.
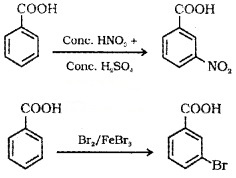
Uses of Carboxylic Acids
- Methanoic acid- in rubber, textile, dyeing, leather and electroplating.
- Ethanoic acid-as a solvent and as vinegar in food industry.
- Hexanedioic acid – in the manufacture of Nylon 6, 6.
- Esters of benzoic acid – in perfumary.
- Sodium benzoate – as food preservative.
- Higher fatty acids – for the manufacture of soaps and detergents.
We hope the Plus Two Chemistry Notes Chapter 12 Aldehydes, Ketones and Carboxylic Acids help you. If you have any query regarding Plus Two Chemistry Notes Chapter 12 Aldehydes, Ketones and Carboxylic Acids, drop a comment below and we will get back to you at the earliest.
