Plus Two Chemistry Notes Chapter 11 Alcohols, Phenols and Ethers is part of Plus Two Chemistry Notes. Here we have given Plus Two Chemistry Notes Chapter 11 Alcohols, Phenols and Ethers.
| Board | SCERT, Kerala |
| Text Book | NCERT Based |
| Class | Plus Two |
| Subject | Chemistry Notes |
| Chapter | Chapter 11 |
| Chapter Name | Alcohols, Phenols and Ethers |
| Category | Plus Two Kerala |
Kerala Plus Two Chemistry Notes Chapter 11 Alcohols, Phenols and Ethers
Alcohols and phenols are formed when a hydrogen atom in hydrocarbon is replaced by -OH group. In alcohols one or more -OH groups are directly attached to carbon atom(s) of an aliphatic system. While phenols contain -OH group(s) directly attached to carbon atom(s) of an aromatic system. Ethers are alkoxy oraryloxy hydrocarbons.
Classification
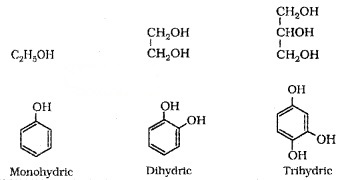
1. Mono, Di, Tri or Polyhydric Compounds:
Alcohols and phenols are classified as mono-di-tri-polyhydric depending upon number of -OH group.
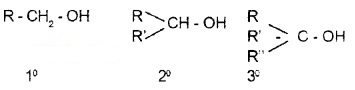
(i) Compounds Containing sp3 – C – OH Bond:
The – OH group is attached to sp3 C. They are further classified as 1°, 2°, and 3°.
Allylic Alcohols:
The -OH group is attached to a sp3 C next to the C = C.
![]()
Benzylic Alcohols:
The -OH group is attached to a sp3 C next to an aromatic ring.
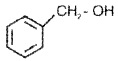
Allylic and benzylic alcohols may be 1°,2° or 3°.
(ii) Compounds Containing sp2 C- OH Bond:
The – OH group bonded to a C = C i.e., to a vinylic or to an aryl C. Vinylic alcohol: CH2 = CH – OH
Ethers:
They are classified as simple ethers or symmetrical ethers – if the alkyl or aryl group attached to O are same and mixed ether or unsymmetrical ether- if the two groups attached to O are different.
Simple ethers
CH3 – O – CH3
C2H5 – O – C2H5
Mixed ethers
CH3 – O – C2H53
C2H5 – O – C3H7
Nomenclature
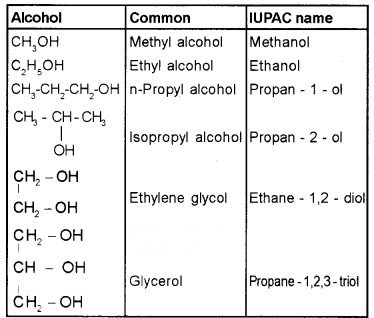
(a) Alcohols: Common name – alkyl alcohols
IUPAC – alkanols (‘e’of the alkane is replaced by ‘ol’)
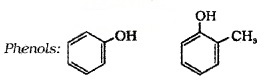
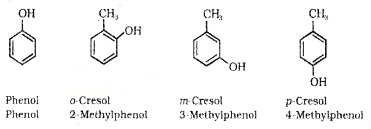
(b) Phenols:
These are hydroxy derivative of benzene. The name phenol is also accepted by IUPAC.
(c) Ethers:
Common name – Alkyl Ether
IUPAC name – Aikoxyalkane
The smaller R – group is chosen as alkoxy and larger R – group is choosen as parent hydrocarbon.

Structure of Functional Group
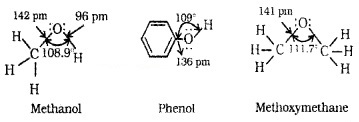
Alcohols:
C of C – OH bond is sp3 hybridised. Bond is formed by sp3 – sp3 overlap. C – OH bond angle slightly less than the tetrahedral bond angle(109°28’) due to the repulsion between the unshared electron pairs of oxygen.
Phenols:
The -OH group is attached to sp2 C of an aromatic ring. The C – O bond length is slightly less than that in methanol. This is due to
- Partial double bond character on account of the conjugation of unshared electron pair of O with the aromatic ring and
- sp2 hybridised state of C to which O is attached.
Ethers:
The 4 electron pairs (2 bond pairs and 2 lone pairs) on O are arranged approximately in a tetrahedral arrangement. The bond angle is slightly greaterthan the tetrahedral angle due to the repulsive interaction between the two bulky -R groups.
Alcohols and Phenols
1. Preparation of Alcohols
(1) From alkenes:
(i) By acid catalysed hydration:
Alkenes react with water in presence of acid as catalyst. The addition is according to Markovnikov’s rule.
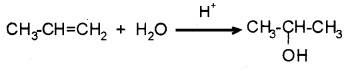
(ii) By hydroboration – oxidation:
Diborane – B2H6 or (BH3)2 reacts with alkene to given trialkyl borane which is oxidised to alcohol by H2O2 in presence of aq. NaOH.
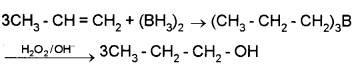
(2) From Carbonyl Compounds:
(i) By Reduction of Aldehydes and Ketones:
These are reduced using LiAlH4, NaBH4, H2Pd etc.
Aldehyde gives 1° alcohols, while ketones gives 2° alcohols.
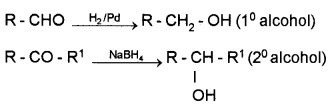
(ii) By the Reduction of Carboxylic Acids or Esters:
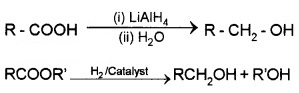
(3) From Griguard Reagents:
By the reaction of Grignard reagents with aldehydes and ketones. The adduct formed by the nucleophilic addition of RMgX to carbonyl group on hydrolysis yeilds alcohol.
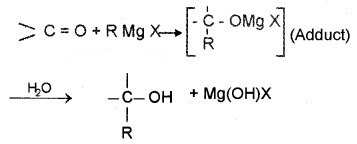
- Formaldehyde (HCHO) gives 1° alcohols
- Other aldehydes (R – CHO) give 2° alcohols
- Ketones (R – CO – R) give 3° alcohols
Example:
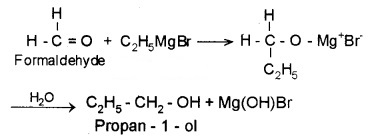
2. Preparation of Phenols:
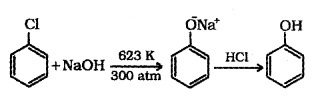
(a) From Hatoarenes:
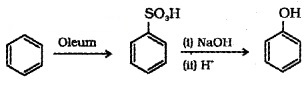
(b) From Benzene Sulphonic Acid:
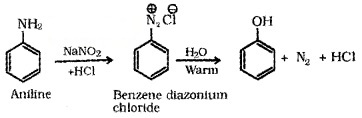
(c) From Diazonium Salts:
(d) From Cumene (Isopropylbenzene):
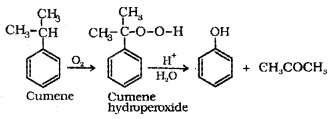
3. Physical Properties:
The boiling points of alcohols and phenols increase with increase in the number of carbon atoms. In alcohols the boiling points decrease with increase of branching in carbon chain. The high boiling point of alcohols is due to intermolecular hydrogen bonding.
Solubility:
Solubility of alcohols and phenols in water is due to their ability to form hydrogen bonding. The solubility decreases with increase in size alkyl/aryl group.
4. Chemical Reactions of Alcohols:
(a) Reactions Involving Cleavage of O-H Bond
(1) Acidity of Alcohol and Phenols:
(i) Reaction with Metals:
Alcohols and phenols react with active metals such as Na, K and Al to yield corresponding alkoxides/phenoxides and H2.
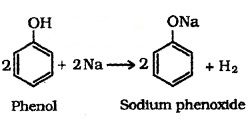
Phenols react with aq. NaOH to form sodium phenoxides.
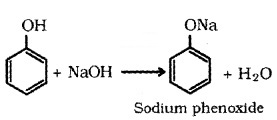
(ii) Acidity of Alcohols:
It is due to the polar nature of O-H bond. Electron releasing groups increase electron density on O tending to decrease the polarity of O-H bond. The acid strength of alcohols decreases in the order: 1°> 2° > 3° alcohols.
(iii) Acidity of Phenols:
The reaction of phenol with aqueous NaOH indicates that phenols are stronger acids than alcohols and water. Acidity of phenols can be explained by resonance.
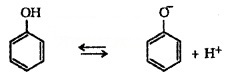
The delocalisation of negative charge makes phenoxide ion more stable and favours the ionisation of phenol.
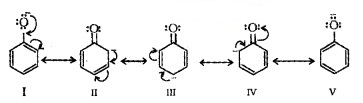
The acidity of phenols increases if an electron-withdrawing group is present at 0- and p- position. Electron releasing groups decrease the acidity.
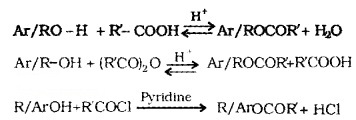
(2) Esterification:
Alcohols and phenols react with carboxylic acids, acid chlorides and acid anhydrides to form esters.
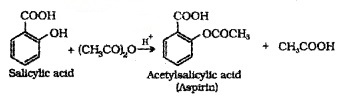
Acefy/afron:
Introduction of acetyl (CH3CO-) group in alcohols or phenols. Acetylation of salicylic acid produces aspirin.
(b) Reaction Involving Cleavage of C-0 Bond in Alcohols 1) Reaction with Hydrogen Halides:
R – OH + HX → R – X + H2O
(1) Lucas Test:
Alcohols are distinguished by Lucas reagent (cone. HCI and ZnCl2). On treating with Lucas reagent, 3° alcohol gives immediate turbidity, 2° alcohol gives turbidity after few minutes, 10 alcohol do not produce turbidity at room temperature.
(2) Reaction with Phosphorus Trihalide (PCl3):
3 R – OH + PCl3 → 3 R – Cl + H3PO3.
(3) Dehydration:
Alcohols undergo dehydration to form alkenes on treating cone. H2SO4 or H3PO4.
![]()
e.g. ethanol undergoes dehydration by heating it with cone. H2SO4 at 443 K.
![]()
The relative ease of dehydration of alcohols in the follows the order 3° > 2° > 1°
(4) Oxidation:
It i nvolves the formation of a
![]()
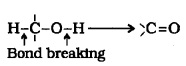
1° alcohols are oxidised to aldehydes.
![]()
Strong oxidising agents such as acidified KMnO4 or K2Cr2O7 are used forgetting carboxylic acids from alcohols directly. A better reagent for oxidation of 1° alcohol to aldehydes is pyridinium chlorochromate (PCC).
![]()
2° alcohols are oxidised to ketones by CrO3.
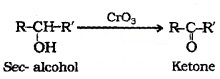
3° alcohols do not undergo oxidation.
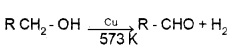
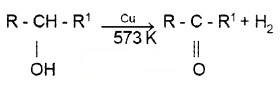
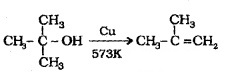
Dehydrogenation:
When the vapours of a alcohols are passed over heated Cu at 573 K,
1° alcohols give addehyde.
2° alcohols give ketones.
3° alcohols undergo dehydration to give alkene.
(c) Reactions of Phenols:
(1) Electrophilic Aromatic Substitution:
The -OH group attached to the benzene ring activates it towards electrophilic substitution. It is 0- and p- directing.
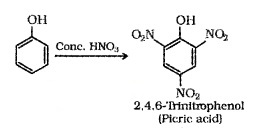
(i) Nitration:
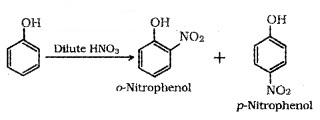
o-Nitrophenol is steam volatile due to intramolecular H – bonding and p-Nitrophenol is less volatile due to intermolecular H -bonding. Hence the mixture can be seperated by steam distillation.
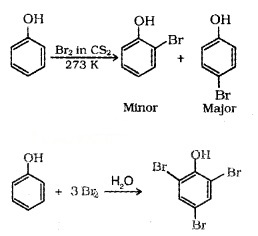
(ii) Halogenation:
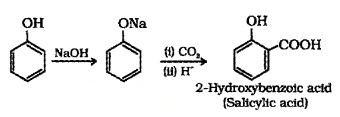
(2) Kolbe’s Reaction:
Phenol, when treated with NaOH, forms sodium phenoxide which undergoes electrophilic substitution with CO2 to give 2- Hydroxybenzoic acid (Salicylic acid).
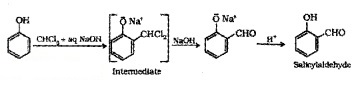
(3) Reimer – Tiemann Reaction:
On treating phenol with CHCI3 in presence of aq. NaOH, 2 – Hydroxy benzaldehyde (Salicylaldehyde) is formed.
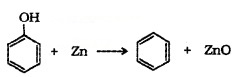
(4) Reaction with Zn Dust:
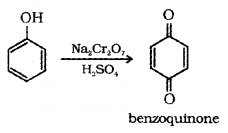
(5) Oxidation:
Some Commercially Important Alcohols
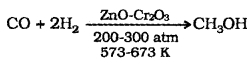
(1) Methanol (CH3 – OH):
It is also known as wood spirit. Industrial preparation – Catalytic hydrogenation of carbon monoxide at high pressure and temperature and in the presence of ZnO-Cr2O3 catalyst.
Methanol is a colourless liquid, poisonous in nature, cause blindness and in large quantities causes even death. It is used as solvent in paints and varnishes.
(2) Ethanol (C3 – CH2 – OH):
Obtained commercially by fermentation of sugars. The enzyme invertase present in the yeast converts sugar into glucose and fructose, which undergo fermentation in presence of zymase, another enzyme found in yeast.
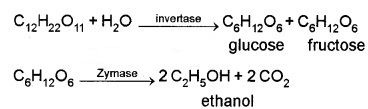
It is a colourless liquid, used in paint industry as a solvent.
Rectified spirit – 95.6% ethanol.
Absolute alcohol – Pure anhydrous alcohol (100% alcohol)
Power alcohol – Alcohol mixed with gasoline (1:4 ratio).
Denatured spirit- The commercial alcohol is made unfit for drinking by mixing in it some CuSO4, pyridine or methanol. It is known as denaturation of alcohol.
Ethers
1. Preparation of Ethers:
(1) By Dehydration of Alcohols:
Alcohols undergo dehydration in presence of protic acids. (H2SO4, H3PO4)
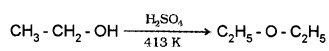
(2) Williamson Synthesis:
Alkyl halides react with sodium alkoxide to form ether.
R – X + R’ONa → R – O – R’ + NaX
Better results are obtained if alkyl halide is primary.
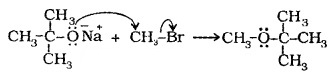
In case of secondary and tertiary akyl halides, elimination competes substitution.
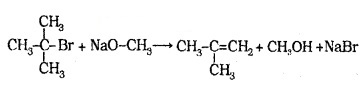
Phenols are also converted to ethers by this method.
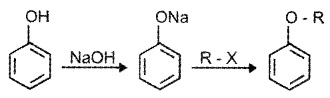
2. Physical Properties:
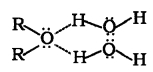
Ethers have much lower boiling points than alcohols. It is due to the presence of H-bonding in alcohols. Lower members of ethers are soluble/miscible in water as they form hydrogen bonds with a water molecules.
3. Chemical Reactions:
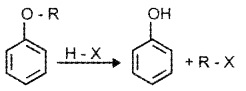
(1) Cleavage ofC-0 Bond in Ethers:
It takes place under drastic conditions with excess of HX.
R – O – R + HX → R – X + R – OH
R – OH + HX → R – X + H2O
Alkyl aryl ethers react with HX to give phenol and alkyl halide.
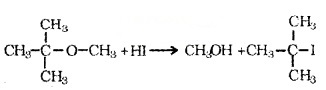
Order of reactivity of hydrogen halides is Hl > HBr > HCI. In the reaction of ether with HI, if the ether contains primary or secondary alkyl groups, it is the lower alkyl group that forms alkyl iodide.
e.g. CH3 – O – CH2CH3l + H – l → CH3l + CH3CH2OH. When one of the alkyl group is a tertiary group, the halide formed is a tertiary halide.
(2) Electrophilic Substitution:
It occurs at o- and p- position as the -OR group is o- and p- directing.
(i) Hologenation:
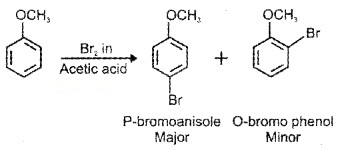
(ii) Nitration:
Anisole reacts with cone. HN03 as follows:
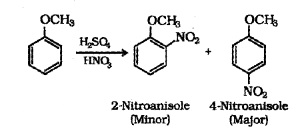
(iii) Friedel-Crafts reaction:
(a) Alkylation:
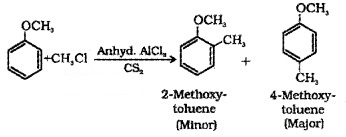
(b) Acetylation:
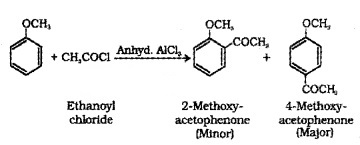
We hope the Plus Two Chemistry Notes Chapter 11 Alcohols, Phenols and Ethers help you. If you have any query regarding Plus Two Chemistry Notes Chapter 11 Alcohols, Phenols and Ethers, drop a comment below and we will get back to you at the earliest.
