Plus Two Chemistry Chapter Wise Questions and Answers Chapter 7 The p Block Elements are part of Plus Two Chemistry Chapter Wise Questions and Answers. Here we have given Plus Two Chemistry Chapter Wise Questions and Answers Chapter 7 The p Block Elements.
Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 7 The p Block Elements
Plus Two Chemistry The p Block Elements One Mark Questions and Answers
Question 1.
The gas obtained by the thermal decomposition of barium azide is _________.
Answer:
Nitrogen
Question 2.
The number of replaceable hydrogen atoms in ortho phosphorous acid (H3PO3) is
(a) 3
(b) 2
(c) 1
(d) 0
Answer:
(b) 2
Question 3.
The stable allotrope of sulphur at room temperature is ___________ .
Answer:
Rhombic sulphur
Question 4.
Which element would readily replace oxygen from an oxide?
(a) N
(b) S
(c) F
(d) Cl
(Answer:
(c) F
Question 5.
The geometry of XeF4 is.
Answer:
Square planar
Question 6.
The shape and hybridisation of XeF4 molecule
Answer:
Square planar and sp3d2
Question 7.
The least stable hydride of 15th group elements is.
Answer:
(BiH3)
Question 8.
Choose the weak monobasic acid among the following.
(a) H3BO3
(b) H3PO2
(c) H3PO4
(d) HNO3
Answer:
(a) H3BO3
Question 9.
The bond enthalpy is highest for ________
Answer:
H2
Question 10.
Magnetic moment of an atom with atomic no. 24 in aqeous solution is ____________.
Answer:
4.90 B.M.
Plus Two Chemistry The p Block Elements Two Mark Questions and Answers
Question 1.
In the class, a student argued that “heterogenous” catalysis and Le-Chatlier’s principles are applied in contact process.”
- Do you agree with this statement?
- Justify.
Answer:
1. Yes
![]()
The reaction is exothermic, reversible and the forward reaction leads to a decrease in volume. Therefore, low temperature and high pressure are the favourable conditions for maximum yield according to Le Chatelier’s principle.
2. Contact process is an example of heterogeneous catalysis since the catalyst V2O5 is solid while the reactants are gases.
Question 2.
ClF3 exists, but FCl3 doesn’t, why?
Answer:
In the valence shell of Cl vacant 3d orbitals are available. Hence it can form ClF3. But, F has no vacant d orbital to show higher oxidation state and FCl3 is not possible.
Question 3.
In a classroom discussion, a student argued that carbon has the maximum catenation property.
- Do you agree?
- What do you understand by the term catenation?
- List out some other elements which can show catenation?
Answer:
- Yes.
- Catenation is the self linking property of an atom. It is maximum for carbon, because C-C bond is very strong.
- Sulphur and Phosphorus.
Question 4.
Some elements and their ores are given in table. Arrange them correctly.
| A | B |
| a) Calcium | Galena |
| b) Potassium | Magnetite |
| c) Lead | Fluorapatite |
| d) Tin | Gypsum |
| e) Phosphorus | Cassiterite |
| Carnallite |
Answer:
| A | B |
| a) Calcium | Gypsum |
| b) Potassium | Carnallite |
| c) Lead | Galena |
| d) Tin | Cassiterite |
| e) Phosphorus | Fluorapatite |
Question 5.
Analyse the statement: ‘Ionisation enthalpy of halogens decreases with a decrease in atomic size’. Is it true? Justify your answer.
Answer:
No. On moving from top to bottom in a given group, the size of the atom increases and hence ionisation enthalpy decreases.
Question 6.
Hydrides of group 15 are Lewis bases.
- Give reason.
- Arrange the group 15 hydrides in the decreasing order of basic strength.
Answer:
- Due to the presence of lone pair on the central atom which is available for donation.
- NH3 > PH3 > ASH3 > SbH3 ≥ BiH3.
Question 7.
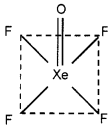
According to VSEPR theory assign structure to XeOF4.
Answer:
XeOF4 possess square pyramidal shape.
Question 8.
Why does the reactivity of nitrogen differ from phosphorus?
Answer:
- Nitrogen has a small size, high electronegativity, high ionisation enthalpy as compared to phosphorus.
- Nitrogen does not contain vacant d-orbitals in its valence shell whereas phosphorus contains vacant d-orbitals in its valence shell.
- Nitrogen has the ability to form triple bond ( N ≡ N) as a result of which its bond enthalpy (941.4 kJ mol-1) is very high making it less reactive.
Question 9.
Why does NH3 form hydrogen bond but PH3 does not?
Answer:
In NH3, the nitrogen atom forms hydrogen bond because of the following reasons:
- Small size of nitrogen
- High electronegativity (3.0) of nitrogen
N-H bond is polar forming hydrogen bond.
P-H bond is almost purely covalent due to larger size and lesser electronegativity.
Question 10.
The HNH angle is higher than HPH, HAsH and HSbH angles. Why?
Answer:
Because in NH3 is sp3 hybridised. Due to lone pair of electrons the bond angle contracts from 109° 28’ to 106.5°. The sp3 hybridisation becomes less and less distinct with increasing size of the central atom. Thus, the bond angle of the hydrides of group 15 decreases as.
![]()
Question 11.
Can PCl3 act as an oxidising as well as a reducing agent? Justify.
Answer:
This is because in PCl3 phosphorus is in the intermediate oxidising state of -3.
1. As reducing agent:
The following reactions support the reducing behaviour of PCl3.
- PCl3 + SO2Cl2 → PCl5 + SO2
- PCl3 + SO3 → POCl3 + SO2
2. As an oxidising agent:
It oxidises metals to their respective chlorides.
- 12Ag + 4PCl3 → 12AgCl + P4
- 6Na + PCl3 → 3NaCl + Na3P
Question 12.
Explain why inspite of nearly the same electronegativity, nitrogen forms hydrogen bonding while chlorine does not.
Answer:
Due to its larger size (99 pm) as compared to oxygen (66 pm).
Question 13.
Ozone is a strong oxidising agent. Why?
Answer:
O3 undergoes dissociation to form nascent oxygen. This nascent oxygen is responsible for oxidising property of ozone.
O3 → O2 + [O]
Question 14.
White phosphorus is more reactive than other solid phases of phosphorus. Give reason.
Answer:
This is beacuse of angular strain in the P4 molecule where the angles are only 60°.
Question 15.
What happens when
- Concentrated H2SO4 is added to CaF2?
- SO3 is passed through water?
Answer:
1. It form hydrogen fluoride.
CaF2 + H2SO4 → CaSO4 + 2HF
2. It dissolves SO3 to give H2SO4.
SO3 + H2O → H2SO4
Question 16.
Give two important fluorides of Xenone. Predict their structure.
Answer:
- XeF2 – linear
- XeF4 – Square planar
- XeF6 – Distorted octahedral
Question 17.
Why is N2 less reactive at room temperature?
Answer:
Due to the presence of triple bond between N atoms N2 has high bond dissociation energy and is less reactive.
Question 18.
Arrange the following in the increasing order of thermal stability.
ASH3, NH3, PH3
Answer:
Thermal stability of hydrides, decreases from nitrogen to bismuth: NH3 > PH3 > AsH3.
Question 19.
The 3 allotropic forms of Pare white, red and black.
- Name the thermodynamically most stable allotrope.
- Which allotrope of P is stored in water?
Answer:
- Black phosphorus
- White phosphorus
Question 20.
Nitrogen and Phosphorus are in the same group. PCI5 is known but NCI5 is not known. Why?
Answer:
In phosphorus vacant d-orbitals are present. It can form pentahalides. But due to absence of d-orbitals nitrogen cannot form NCI5.
Question 21.
Why is helium used in diving apparatus?
Answer:
Helium is used in diving apparatus because of its very low solubility in blood.
Question 22.
Why has it been difficult to study the chemistry of radon?
Answer:
Radon is radioactive with very short half-life which makes the study difficult.
Question 23.
Account for the following:
SO3 is more covalent than SO2.
Answer:
Due to high charge and small size of sulphur in +6 oxidation state in SO3 it is more covalent than SO2, in which sulphur is in +4 oxidation state.
Question 24.
I2 is more soluble in KI than in water.
Answer:
I2 combines with KI to form the soluble complex, KI3.
KI + I2 → KI3
Question 25.
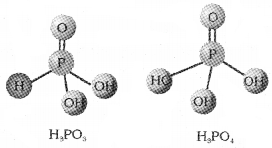
H3PO3 is dibasic while H3PO4 is tribasic.
Answer:
There are three ‘P-OH’ bonds that are ionisable in H3PO4. So, it is tribasic. In H3PO3 there are only 2 ionisable ‘P-OH’ bonds. Hence it is dibasic.
Plus Two Chemistry The p Block Elements Three Mark Questions and Answers
Question 1.
Consider the given reaction:
2A + 6SiO2 → 6CaSiO3 + B
B + 10C → D + 10CO
- Identify A, B and D.
- D is stored underwater. Why?
- Give two examples of oxoacids of D and compounds of D.
Answer:
1. A – Ca3(PO4)2
B – P4O10
D – P4
2. Because P4 readily catches fire in air.
3. Two examples of oxoacids of D and compounds of D
- Oxoacids of D:
Oxoacids – Phosphorous acid(H3PO3), Phosphoric acid (H3PO4). - Compounds of D:
phosphine (PH3), PCI5, PCI4, P4O6
Question 2.
- Suggest a method for the preparation of PCI3.
- Why does PCI3 fume in moist air?
Answer:
1. By passing dry chlorine overheated white phosphorus.
P4 + 6CI2 → 4PCI3
2. PCI3 hydrolyses in the presence of moisture giving fumes of HCI.
PCI3 + 3H2O → H3PO3 + 3HCI
Question 3.
A student argued that electronegativity of p-block elements decrease along period and increases down the group.
- Do you agree with this? Explain.
- Write about the metallic character of p-block elements.
- Arrange the following of p-block elements in the order of decreasing oxidising power.
F2/F-( E° =+2.85 V), Br/Br–( E° =+1.07 V), CI2/CI– (E° =+1.36V), I2/I–( E° =+0.57V).
Answer:
- No. electronegativity of p-block elements increases along a period and decreases down a group.
- Most of the p-block elements are non-metallic in nature. Among p-block elements the metallic character decreases along a period and increases down a group.
- F2 > CI2 > Br2 > I2, because the standard electrode potential values decreases in the same order.
Question 4.
The shape and hybridisation of some interhalogen compounds are given below in wrong order. Match them correctly.

Answer:

Question 5.
Account for the following:
- H2O is a liquid while H2S is a gas.
- H2S is more acidic than H2O
- SF6 is known while SH6 is not known.
Answer:
- There is intermolecular hydrogen bonding in H2O molecule but there is no hydrogen bonding in H2S .
- The S-H bond is weaker than O-H bond because size of S atom is greater than that of O atom. Hence H2S can dissociate to give H+ ions in aqueous solution.
- In the higher oxidation state, S can combine only with highly electronegative elements like F.
Question 6.
Sulphur forms many allotropes such as a – sulphur, β -sulphur etc.
- What do you mean by allotropy?
- How can you convert a – sulphur to b-sulphur?
Answer:
- Certain elements can exist in different forms with different physical property and same chemical properties.
- b -sulphur is prepared by melting a -sulphur in a dish and cooling, till crust is obtained. Holes are then pierced into the crust, and the liquid is taken out. On removing the crust, needle shaped β -sulphur is obtained.
Question 7.
Account for the following:
- PH3 has lower boiling point than NH3.
- Pentahalides are more covalent than trihalides.
- ICI is more reactive than I2.
Answer:
- NH3, PH3 molecules are not associated through intermolecular hydrogen bonding in liquid state.
- Higher the positive oxidation state of central atom, more will be its polarising power which, in turn, increases the covalent character.
- Bond energy of I-CI bond is less than that fo I-I bond.
Plus Two Chemistry The p Block Elements Four Mark Questions and Answers
Question 1.
My name is ‘X’. I am a poisonous colourless gas with the smell of rotten fish.
- Identify ‘X’.
- Explain the laboratory preparation of X.
Answer:
- Phosphine(PH3).
- By heating white phosphorus with concentrated NaOH solution in an inert atmosphere of CO2.
![]()
Question 2.
- How is bleaching powder prepared?
- Give the composition of bleaching powder.
Answer:
1. By treating Cl2 with dry slaked lime.
2Ca(OH)2 + 2CI2 → Ca(OCI)2 + CaCI2 + 2H2O
2. Ca(OCI)2 CaCI2.Ca(OH)2.2H2O.
Question 3.
- Why do noble gases form compounds with fluorine and oxygen only?
- Does the hydrolysis of XeF6 lead to a redox reaction?
Answer:
1. Fluorine and oxygen are small atoms with high value of electronegativity. Fluorine is also highly reactive in nature. It is for this reason they form compounds with noble gases.
2. No, the products of hydrolysis are XeOF4 and XeO2F2 where the oxidation states of all the elements remain the same as it was in the reacting state.
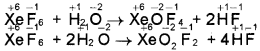
Question 4.
- Interhalogen compounds are more reactive than halogens. Why?
- Is there any exception to the above generalisation. Explain.
Answer:
- This is because the bond in the interhalogen (X – X’) is weaker than X – X bond in the halogens.
- Yes. F2 is more reactive than interhalogen compounds. This is because the F – F bond is weaker than X – X’ bond in interhalogen compounds.
Question 5.
Nitrogen cannot extend its covalency beyond four but it forms wide variety of oxides and also forms oxoacids.
- Name the two oxoacids of nitrogen.
- Action of nitric acid with metals depends on many factors. Justify.
Answer:
1. HNO2, HNO3
2. The product of oxidation depends upon the concentration of the acid, temperature and the nature of the material undergoing oxidation, e.g.
a. 3Cu + 8HNO3(dilute) → 3Cu(NO3)2 + 2NO + 4H2O
Cu + 4HNO3(conc.) → Cu(NO3)2 + 2NO2 + 2H2O
b. Zinc reacts with dilute nitric acid to give N2O and with concentrated acid to give NO2.
4Zn + 4HNO3(dilute) → 4Zn(NO3)2 + 5H2O + N2O
Zn + 10HNO3(conc.) → Zn(NO3)2 + 2H20 + 2NO2
Some metals (e.g., Cr, Al) do not dissolve in concentrated nitric acid due to the formation of a passive film of oxide on the surface.
Question 6.
- Arrange hydrides of group 16 in the order of increasing acidic strength.
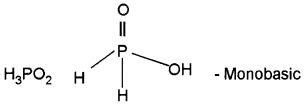
- Draw the structures of any three oxoacids of phosphorus and find out their basicity.
Answer:
1. H2O < H2S < H2Se < H2Te
2.
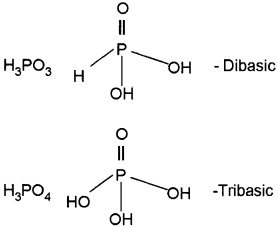
Question 7.
- Noble gases have very low boiling points. Why?
- XeF2 and XeF4 are important Xe compounds. How can we prepare them and what is the action of water on them?
Answer:
1. Noble gases being monoatomic have no interatomic forces except weak dispersion forces and therefore, they are liquefied at very low temperatures. Hence, they have low boiling points.
2. XeF2 and XeF4 are formed by the direct reaction of elements under the specific conditions.
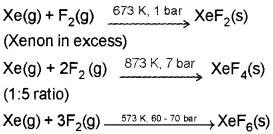
They are readly hydrolysed even by traces of water,
e.g. 2XeF2(s) + 2H2O(l) → 2Xe(g) + 4HF(aq) + O2(g)
Question 8.
It is greenish yellow gas with an offensive smell used in water purification. It partially dissolves in water to give a solution which turns blue litmus to red. When it is passed through NaBr solution bromine is formed.
- Identify the gas.
- Identify the group to which it belongs.
- Write the electronic configuration.
- Write the equation showing its reaction with water.
Answer:
- Chlorine
- Halogen family (17th group)
- 1s2 2s2 2p6 3s2 3p5
- CI2 + H2O → HCI + HOCI
Question 9.
Basic character of hydrides of group 15 is due to the presence of lone pair on the central atom.
- NH3 is strongly basic while BiH3 is weakly basic. Why?
- Differentiate between allotropic forms of phosphorus.
Answer:
1. Due to the presence of lone pair of electrons on nitrogen atom of NH3. But in BiH3, due to the large size of Bi the electron density decreases and hence is less basic.
2. The allotropic forms of P are White, Red and Black phosphorus. White phosphorus consists of tetrahedral P4 molecules. Red phosphorus is polymeric in structure consisting of chains of P4 tetrahedra linked together. Black phosphorus has a layer type structure and has α and β forms.
Question 10.
- Give a method for preparation of XeO3.
- Deduce the molecular shape of BrF3 on the basis of VSEPR theory.
Answer:
1. XeO3 is prepared by hydrolysis of xenon hexa fluride in presence of water.
XeF6 + 3H20 → XeO3 + 6HF
2. In BrF3 the central atom Br is sp3d hybridised with 3 bond pairs and 2 lone pairs. According to VSEPR theory the expected geometry is trigonal bipyramidal. But due to strong Ip – Ip and Ip – bp repulsions compared to weak bp – bp repulsion it Assumes a bend T – shape as shown below
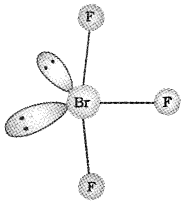
Question 11.
Account for the following:
- BiH3 is the strongest reducing agent among all the hydrides of group 15 elements.
- Bleaching action of chlorine.
Answer:
- It is because BiH3 is least stable among the hydrides of group 15 elements.
- Bleaching action of chlorine is due to oxidation. The nacent oxygen ([O]) produced is responsible for the bleaching action.
CI2 + H2O → 2HCI + [O]
Coloured substance + [O] → Colourless substance
Plus Two Chemistry The p Block Elements NCERT Questions and Answers
Question 1.
- Noble gases have very low boiling points. Why?
- XeF2 and XeF4 are important Xe compounds. How can we prepare them and what is the action of water on them?
Answer:
1. Noble gases being monoatomic have no interatomic forces except weak dispersion forces and therefore, they are liquefied at very low temperatures. Hence, they have low boiling points.
2. XeF2 and XeF4 are formed by the direct reaction of elements under specific conditions.
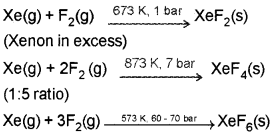
They are readly hydrolysed even by traces of water,
e.g. 2XeF2(s) + 2H2O(l) → 2Xe(g) + 4HF(aq) + O2(g)
Question 2.
Why does the reactivity of nitrogen differ from phosphorus?
Answer:
- Nitrogen has a small size, high electronegativity, high ionisation enthalpy as compared to phosphorus.
- Nitrogen does not contain vacant d-orbitals in its valence shell whereas phosphorus contains vacant d-orbitals in its valence shell.
- Nitrogen has the ability to form triple bond ( N ≡ N) as a result of which its bond enthalpy (941.4 kJ mol-1) is very high making it less reactive.
Question 3.
Why does NH3 form hydrogen bond but PH3 does not?
Answer:
In NH3, the nitrogen atom forms hydrogen bond because of the following reasons:
- Small size of nitrogen
- High electronegativity (3.0) of nitrogen
Due to more difference of electronegativity between N and H atom the N-H bond is polar forming hydrogen bond. On the contrary, in PH3 the P-H bond is almost purely covalent due to larger size and lesser electronegativity (2.11) of phosphorus and hence does not form hydrogen bond.
Question 4.
The HNH angle is higher than HPH, HAsH and HSbH angles. Why?
Answer:
Because in NH3 is sp3 hybridised. Due to lone pair of electrons the bond angle contracts from 109° 28’ to 106.5°. The decreased bond angle in other hydrides is because of the fact that the sp3 hybridisation becomes less and less distinct with increasing size of the central atom i.e., pure p-orbitals are utilised in M-H bonding or in simple words the s- orbital of H atom overlaps with orbital having almost pure p-character. Thus, the bond angle of the hydrides of group 15 decreases as
![]()
Question 5.
Can PCI3 act as an oxidising as well as a reducing agent? Justify.
Answer:
Yes, This is because in PCI3 phosphorus is in the intermediate oxidising state of -3.
1. As an reducing agent:
The following reactions support the reducing behaviour of PCI3.
- PCl3 + SO2Cl2 → PCl5 + SO2
- PCl3 + SO3 → POCl3 + SO2
2. As an oxidising agent:
It oxidises metals to their respective chlorides.
- 12Ag + 4PCl3 → 12AgCl + P4
- 6Na + PCl3 → 3NaCl + Na3P
Question 6.
Explain why inspite of nearly the same electronegativity, oxygen forms hydrogen bonding while chlorine does not.
Answer:
Oxygen atom can form hydrogen bond whereas chlorine does not. The tendency for hydrogen bonding depends upon
- Small size and
- High electronegativity values
Although the electronegativities of O and Cl are nearly the same yet chlorine does not form hydrogen bond due to its larger size (99 pm) as compared to oxygen (66 pm).
We hope the given Plus Two Chemistry Chapter Wise Questions and Answers Chapter 7 The p Block Elements will help you. If you have any query regarding Plus Two Chemistry Chapter Wise Questions and Answers Chapter 7 The p Block Elements, drop a comment below and we will get back to you at the earliest.
