Plus Two Chemistry Chapter Wise Questions and Answers Chapter 6 General Principle and Processes of Isolation of Elements are part of Plus Two Chemistry Chapter Wise Questions and Answers. Here we have given Plus Two Chemistry Chapter Wise Questions and Answers Chapter 6 General Principle and Processes of Isolation of Elements.
Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 6 General Principle and Processes of Isolation of Elements
Plus Two Chemistry General Principle and Processes of Isolation of Elements One Mark Questions and Answers
Question 1.
Siderite is chemically _______________.
Answer:
Iron carbonate (Fe2CO3)
Question 2.
A mineral is called an ore if
(a) The metal present in the mineral is costly
(b) A metal can be extracted from it
(c) A metal can be profitably extracted from it
(d) A metal cannot be extracted from it.
Answer:
(c) A metal can be profitably extracted from it
Question 3.
Predict whether the following statement is true or false? Calcination is done in presence of plenty of air.
Answer:
false
Question 4.
The Ellingham diagram is a plot of
(a) ∆fG° vs T
(b) ∆fH° vs T
(c) ∆fS° vs T
(d) ∆fG° vs ∆fH°
Answer:
(a) ∆fG° vs T
Question 5.
Which of the following metals can be refined using van Arkel method?
(a) Ni
(b) Si
(c) Cu
(d) Zr
Answer:
(d) Zr
Question 6.
Arrange the five elements which together constitute more than 90% of earth’s crust in the decreasing order of their abundance.
Answer:
Oxygen > Silicon > Aluminium > Iron > Calcium.
Question 7.
Suggest the method for the refining of following metals.
- Copper
- Germanium
- Zirconium
Answer:
- Copper – Electrolytic refining
- Germanium – Zone refining
- Zirconium – van Arkel method
Question 8.
Which one of the following does not occurs as sulphide ore
(a) Zn
(b) Cr
(c) Ag
(d) Fe
(e) Hg
Answer:
(b) Cr
Question 9.
Refining of zirconium is by __________________ method
Answer:
Van Arkel method.
Question 10.
Sphalerite is concentrated by ___________.
Answer:
Froth floation
Question 11.
Litharge is an ore of ___________.
Answer:
Lead
Question 12.
The process used for the extraction of sodium is
Answer:
Down’s process
Plus Two Chemistry General Principle and Processes of Isolation of Elements Two Mark Questions and Answers
Question 1.
Explain the terms
Calcination and Roasting with example.
Answer:
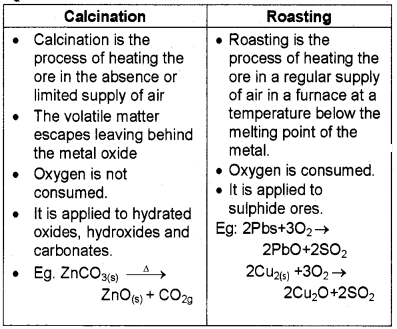
Question 2.
Complete the table:
| A | B |
| 1. Iron | Haematite |
| 2. Sodium | ………………… |
| 3. Chromium | …………………. |
| 4 …………….. | SnO2 |
| 5 …………….. | CuFeS2 |
Answer:
| A | B |
| 1. Iron | Haematite |
| 2. Sodium | Rock salt |
| 3. Chromium | Chromite ore |
| 4. Tin | SnO2 |
| 5. Copper | CuFeS2 |
Question 3.
Why is the reduction of a metal oxide easier if the metal formed is in liquid state at the temperature of reduction?
Answer:
The entropy is higher if the metal is in liquid state than in solid state. The value of (∆S) of the reduction process is +ve when the metal formed is in liquid state the metal oxide being reduced is in solid state. The value of ∆GΘ becomes more on -ve side and the reduction becomes easier.
Question 4.
Before final metallurgical operations the concentrated ore is subjected to some preliminary chemical treatments. Two processes employed for this purpose are carried out in reverberatory furnace.
- Name the two processes.
- To which form the ore is converted through these processes?
Answer:
- Calcination and Roasting
- In both processes ore is converted into oxide form.
Question 5.
Match the following:
| Process | Metal Purified |
| 1) Mond’s Process | Zirconium |
| 2) van Arkel process | Silicon |
| 3) Zone refining | Zinc |
| 4) Distillation | Nickel |
Answer:
| Process | Metal Purified |
| 1) Mond’s Process | Nickel |
| 2) van Arkel process | Zirconium |
| 3) Zone refining | Silicon |
| 4) Distillation | Zinc |
Question 6.
What is the Ellingham diagram? Mention its application.
Answer:
It is a graph showing the variation of ∆rG°forthe formation of oxides with temperature. It helps in the choice of reducing agent in the reduction of oxides.
Question 7.
Although thermodynamically feasible, in practice, magnesium metal is not used for the reduction of alumina in the metallurgy of Aluminium. Why?
Answer:
The process would be uneconomic because Mg itself is a costly metal. Moreover, there is one technological difficulty also. The reaction between Mg and Al2O3 is exothermic. If the temperature increases to 2000 K then the reverse reaction becomes feasible, i.e., Al starts reducing MgO.
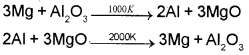
Question 8.
Distinguish between mineral and ore.
Answer:
- Mineral: Various compounds of metals which are found in earth’s crust.
- Ores: The minerals from which metal can be easily and economically extracted.
Question 9.
Which flux can be used to remove a metal oxide impurity from a sulphide ore of noble metal? Substantiate.
Answer:
Silica, SiO2. Generally, metal oxides are basic in nature. To remove basic impurities an acidic flux like SiO2 is used.
Question 10.
Match the following:
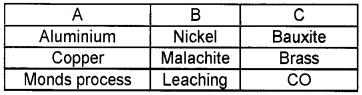
Answer:
- Aluminium – Leaching – Bauxite
- Copper – Malachite – Brass
- Mond’s process – Nickel – CO
Question 11.
Match the following:
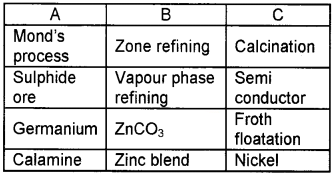
Answer:
- Mond’s process – Vapour phase refining – Nickel
- Sulphide ore – Zinc blende – Froth floatation
- Germanium – Zone refining – Semiconductor
- Calamine – ZnCO3 – Calcination
Question 12.
Differentiate Cast Iron and pig iron.
Answer:
1. Cast iron:
- It is a form of iron obtained from pig iron.
- lt has 3% carbon content.
2. Pig iron:
- It is the least pure form of iron obtained directly from the blast furnace.
- It contains about 4% carbon and many impurities in smaller amount.
Question 13.
How is leaching carried out in case of low grade copper ores?
Answer:
Copper is leached out using acid or bacteria. The solution containing Cu2+ ions is treated with iron scrap or H2 to recover copper.
Cu2+(aq) + Fe(s) → Cu(s) + Fe2+(aq)
Cu2+(aq) + H2(g) → Cu(s) + 2H+(aq)
Question 14.
Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction?
Answer:
Carbon is a poor reducing agent for sulphide ores whereas it is good reducing agent for oxide ores.
Question 15.
What is the role of graphite rod in the electrometallurgy of aluminium?
Answer:
Graphite rod acts as anode in the electrometallurgy of aluminium. Graphite anode facilitates reduction of Al2O3 to aluminium by electrolysis. Carbon reacts with oxygen liberated at anode producing CO and CO2
Question 16.
Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present?
Answer:
The elements antimony, selenium, gold, silver, platinum, etc. are present in the anode mud during refining of copper. These impurities being less electropositive do not undergo oxidation at anode and hence settle down as such.
Plus Two Chemistry General Principle and Processes of Isolation of Elements Three Mark Questions and Answers
Question 1.
The following are some ores. Calamine (ZnCO3), Haematite (Fe2O3), Cinnabar (HgS), Bauxite (Al2O3.2H2O)
- Which ore is concentrated by froth floating process?
- How is Haematite concentrated?
- Which of the ores is concentrated by leaching?
Answer:
- Cinnabar (HgS). Sulphide ores are concentrated by this process.
- By magnetic separation.
- Bauxite (Al2O3.2H2O)
Question 2.
Some data are given below:
(Iron tank, Carbon lining, Cryolite, Carbon blocks, Electricity)
- Identify the metal whose metallurgy is associated here.
- Explain the extraction of this metal.
Answer:
- Aluminium.
- The alumina is dissolved in a mixture of molten cryolite. It is then electrolysed in a rectangular steel tank, with carbon lining, which serves as cathode. Anode is a set of thick carbon rods suspended from top into the fused Al2O3. The temperature is maintained as 1200 Kand 1310 K. Oxygen is evolved at anode which reacts with carbon of anode producing CO and CO2. Aluminium formed at the cathode gets collected.
Question 3.
- What is the role of cryolite in the metallurgy of aluminium?
- Match the following :
| Metal | Process |
| 1. Al | Mond’s process |
| 2. Si | van Arkel process |
| 3. Zr | Zone refining |
| 4. Ni | Leaching |
Answer:
1. Cryolite is used as a solvent to dissolve alumina.
2.
- Al → Leaching
- Si → Zone refining
- Zr → van Arkel process
- Ni → Mond’s process
Question 4.
- Name the chief ores of Aluminium and Iron.
- What methods are employed for the concentration of these ores?
Answer:
1. The chief ores of Aluminium and Iron
- Al → Bauxite
- Iron → Haematite
2. Bauxite is concentrated by leaching and haematite is concentrated by magnetic separation.
Question 5.
The choice of reducing agents in a particular case depends on the thermodynamic factor.
- How far do you agree with this statement?
- Support your opinion with an example.
Answer:
- The statement is true. Choice of reducing agents depends strongly on factors like ΔH, ΔS, ΔG and T for the formation of the oxide to be reduced.
- Electropositive metals like Al, K etc. can be extracted using electricity. Whereas CO is used for reducing haematite in the extraction of iron.
Question 6.
You are provided with samples of impure copper and germanium.
- Which method would you recommend for the purification of each of these metals?
- What is ‘‘Copper matte”? How is it formed?
Answer:
- Coper – Electrolytic refining
Germanium – Zone refining - The copper in the furnace that contains Cu2S and FeS is called copper matte. It is formed when copper ore is heated in a reverberatory furnace after mixing with silica.
Question 7.
As a part of a field trip, students visited a metallurgical plant. They saw that metal is heated in a slopping floor of the furnace.
- Give the name of this process.
- Which type of metals are purified by this method?
- Give example.
Answer:
- Liquation
- Metals with low melting point
- Lead, Tin etc.
Question 8.
Blast furnace produces molten iron which contains impurities such as carbon and sulphur. To make steel, oxygen is blown into the surface of the molten iron. Other elements are then added to give the type of steel required.
- What is slag?
- Name the two gases formed when oxygen reacts with the impurities.
- Name one element which is added to iron to make steel.
Answer:
- Slag is a substance formed by the reaction of impurities with flux.
- Carbon dioxide and Sulphur dioxide.
- Carbon.
Question 9.
What do you mean by refining? Mention the methods also.
Answer:
The process of removal of impurities from the crude metal is called refining. The methods are:
- Distillation
- Liquation
- Electrolytic refining
- Zone refining
- Van Arkel process
- Mond’s process
- Chromatographic methods
Question 10.
Copper can be extracted by hydrometallurgy but not zinc. Explain.
Answer:
Metals occupying low positions in the electrochemical series can be extracted by hydrometallurgy. The metals occupying higher positions in the electrochemical series cannot be extracted by hydrometallurgy because such metal ions are difficult to be reduced.
Copper can be extracted by hydrometallurgy because it occupies lower position in the electrochemical series but Zn occupies higher position.
Plus Two Chemistry General Principle and Processes of Isolation of Elements Four Mark Questions and Answers
Question 1.
The metals such as Ge, Ga, Si etc. are used as semiconductors. So they are to be obtained at high degree of purity.
- Name the method to obtain highly pure Si.
- Ti is purified by using I2. Name the process.
- What is Mond’s process?
Answer:
- Zone refining.
- van Arkel Process.
- For the refining of nickel. In this process, nickel is heated in a stream of CO forming a volatile complex, Ni(CO)4. It is decomposed at high temperature giving pure nickel.
Ni + 4CO → Ni(CO)4 → Ni + 4CO
Question 2.
Some ores are given below:
(ZnS, Al2O3, Fe2O3,Cu2S)
Make a table containing ores, methods of concentration, name of the metal and alloy of the metal.
Answer:
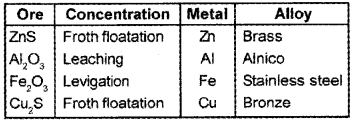
Question 3.
- The value of enthalpy of formation for Cr2O3 is -540 kJ/mol and that of Al2O3is -827 kJ/mol. Is the reduction of Cr2O3 possible with Al?
- Name the metallurgical refining techniques used for Ge and Ni.
Answer:
1. Yes. From the enthalpy of formation values of the concerned oxides it is celar that Al is a strong reducing agent than Cr.
2.
| Element | Metallurgical technique |
| Ge | Zone refining |
| Ni | Mond’s process |
Question 4.
- What is the importance of Ellingham diagram?
- Using the following Ellingham diagram select the suitable reducing agents that can be used for the reduction of Fe2O3 in blast furnace above and below 1000 K.
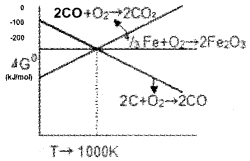
Answer:
1. Ellingham diagram help us in predicting the feasibility of thermal reduction of ore. The criteria is that at a given temperature Gibbs energy of reaction should be negative.
2. Below 1000 K CO is a good reducing agent while above 1000 K carbon is a good reducing agent. This is because below 1000 K the (CO, CO2) line is below the (Fe, FeO) line. But, above 1000 Kthe (C, CO2) line is below the (Fe, FeO) line.
Question 5.
Bauxite is ore of Aluminium.
- What do you mean by an ore?
- Name the method which is used to purify Bauxite.
- Write two examples for ores and their purification methods.
Answer:
1. The mineral from which metal can be easily and economically extracted is called ore.
2. Leaching
3. Two examples for ores and their purification methods
- Hematite → Magnetic separation
- Cinnabar → Froath floatation
Question 6.
- What is the role of depressant in froth floatation process?
- Explain with an example.
Answer:
- Depressants prevent certain type of particles from forming froth during froth floatation process.
- NaCN acts as a depressant for ZnS but not for PbS. Thus, when an ore containing PbS and ZnS is subjected to froth floatation process NaCN selectively prevents ZnS from coming to the froth but allows PbS to come with the froth. In this way, PbS can be separated from ZnS.
Question 7.
- Which ore is used for the extraction of Al?
- What do you mean by extraction of Aluminium?
- Explain the process of purification of ore with chemical equations.
Answer:
- Bauxite
- Removal of earthy impurities (gangue) from bauxite ore and separation of metallic aluminium is called extraction of aluminium.
- Bauxite is treated with NaOH solution and sodium meta aluminate is formed. The aluminate solution is neutralised by passing CO2 gas and hydrated Al2O3 is precipitated by seeding with freshly prepared samples of hydrated Al2O3. Hydrated alumina is filtered, dried and heated to obtain pure Al2O3
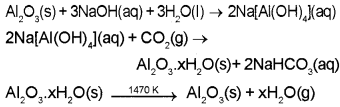
Plus Two Chemistry General Principle and Processes of Isolation of Elements NCERT Questions and Answers
Question 1.
Copper can be extracted by hydrometallurgy but not zinc. Explain.
Answer:
Metals occupying low positions in the electrochemical series can be extracted by hydrometallurgy because the metal ions (Mn+) of such metals can be easily reduced by treatment with some more electropositive metal. The metals occupying higher positions in the electrochemical series cannot be extracted by hydrometallurgy because the metal ions of such metals are difficult to be reduced.
Copper can be extracted by hydrometallurgy because it occupies quite lower position in the electrochemical series. On the other hand, zinc cannot be extracted by hydrometallurgy because it occupies higher position in the series and has large negative reduction potential.
Question 2.
How is leaching carried out in case of low grade copper ores?
Answer:
Copper is leached out using acid or bacteria. The solution containing Cu2+ ions is treated with iron scrap or H2 to recover copper.
Cu2+(aq) + Fe(s) → Cu(s) + Fe2+(aq)
Cu2+(aq) + H2(g) → Cu(s) + 2H+(aq)
Question 3.
Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction?
Answer:
Carbon is a poor reducing agent for sulphide ores whereas it is good reducing agent for oxide ores.
Question 4.
What is the role of graphite rod in the electrometallurgy of aluminium?
Answer:
Graphite rod acts as anode in the electrometallurgy of aluminium. Graphite anode facilitates reduction of Al2O3 to aluminium by electrolysis. Carbon reacts with oxygen liberated at anode producing CO and CO2
At anode:
C (solid) + O2- (melt) → CO(g) + 2e–
C(solid) + 2O2-(melt) → CO2(g) + 4e–
At cathode:
Al3+(melt + 3e– → Al(I)
Question 5.
Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present?
Answer:
The elements antimony, selenium, gold, silver, platinum, etc. are present in the anode mud during the refining of copper. These impurities being less electropositive do not undergo oxidation at the anode and hence settle down as such.
We hope the given Plus Two Chemistry Chapter Wise Questions and Answers Chapter 6 General Principle and Processes of Isolation of Elements will help you. If you have any query regarding Plus Two Chemistry Chapter Wise Questions and Answers Chapter 6 General Principle and Processes of Isolation of Elements, drop a comment below and we will get back to you at the earliest.
