Plus Two Chemistry Chapter Wise Questions and Answers Chapter 5 Surface Chemistry are part of Plus Two Chemistry Chapter Wise Questions and Answers. Here we have given Plus Two Chemistry Chapter Wise Questions and Answers Chapter 5 Surface Chemistry.
Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 5 Surface Chemistry
Plus Two Chemistry Surface Chemistry One Mark Questions and Answers
Question 1.
The process of removing an adsorbed substance from a surface on which it is adsorbed is called ___________.
Answer:
desorption
Question 2.
Which of the following statements is wrong?
(a) Physical adsorption of a gas is directly related to its critical temperature.
(b) Chemical adsorption decreases regularly as the temperature is increased.
(c) Adsorption is an exothermic process.
(d) A solid with a rough surface is a better adsorbent than the same solid with a smooth surface.
Answer:
(c) Adsorption is an exothermic process.
Question 3.
The substances that enhance the activity of a catalyst are called _____________.
Answer:
promoters
Question 4.
The enzyme present in malt which converts starch to maltose is ____________.
Answer:
diastase
Question 5.
The movement of colloidal particles under an applied electric potential is called ________________.
Answer:
electrophoresis
Question 6.
Hair cream is an example of ____________.
Answer:
Emulsion
Question 7.
Gold solution can be prepared by
Answer:
Reduction of gold (III) Chloride with formalin/ Bredig’s method
Question 8.
The correct ascending order of adsorption of the following gases on the same mass of charcoal at the same temperature and pressure is
(a) CH4 < H2 < SO2
(b) H2 < CH4 < SO2
(c) SO2 < CH4 < H2
(d) H2 < SO2 < CH4
Answer:
(b) H2 < CH4 < SO2
Question 9.
In the adsorption of a gas on solid, Freundlich iso-therm is obeyed. The slope of the plot is zero. Then the extent of adsorption is _________.
Answer:
Independent of pressure.
Question 10.
Adsorption is accompanied by _______ in enthalpy and _______ in entropy.
Answer:
decrease, decrease.
Plus Two Chemistry Surface Chemistry Two Mark Questions and Answers
Question 1.
Fill in the blanks.
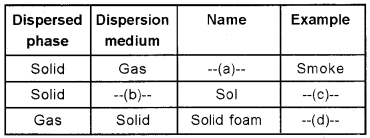
Answer:
Question 2.
Some facts related to adsorption are given below. Classify them into those suitable for Physisorption and Chemisorption.
- Weak attractive force between adsorbent and adsorbate.
- Strong attractive force between adsorbent and adsorbate.
- Reversible process.
- Irreversible process.
- Multilayers are formed.
- Monolayer is formed.
- Heat of adsorption is 20-40 kJ/mol
- Heat of adsorption is 40-400 kJ/mol
Answer:
| Physical adsorption | Chemical adsorption |
| 1. Weak attractive force between adsorbent and adsorbate. | 2. Strong attractive force between adsorbent and adsorbate. |
| 3. Reversible process. | 4. Irreversible process. |
| 5. Multilayers are formed. | 6. Monolayer is formed. |
| 7. Heat of adsorption is 20-40 kJ/mol. | 7. Heat of adsorption is 20-40 kJ/mol. |
Question 3.
What is the influence of temperature in physical and chemical adsorption? Explain it with the help of a graph.
Answer:
In the case of physical adsorption, rate of adsorption x/m is inversely proportional to temperature.
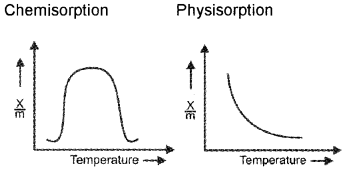
In the case chemical adsorption, rate of adsorption intially increases with increase in temperature, reaches a maximum value and thereafter decreases with increase in temperature.
Question 4.
‘Activity’ and ‘Selectivity’ are two terms related to solid catalysts. Distinguish between these two terms with proper illustrations.
Answer:
- Activity – It is ability of the catalyst to give the product easily.
- Selectivity – It is the ability of a catalyst to direct to yield a particular product, e.g. starting with H2 and CO, and using different catalysts, we get different products.
Question 5.
Give reason why a finely divided substance is more effective as an adsorbent.
Answer:
Adsorption is a surface phenomenon. Since finely divided substance has large surface area, hence, adsorption occurs to a greater extent.
Question 6.
What do you understand by activation of adsorbent? How is it achieved?
Answer:
Activation of adsorbent increases the adsorption power of the adsorbent. By increasing the surface area of the adsorbent,
- Removing the gases already adsorbed and
- Making the surface of adsorbent rough.
Question 7.
What role does adsorption play in heterogeneous catalysis?
Answer:
In heterogeneous catalysis, the reactants are generally gases whereas catalyst is a solid. The reactant molecules are adsorbed on the surface of the solid catalyst. As a result, the concentration of the reactant molecules on the surface increases and hence the rate of reaction increases.
Question 8.
Write notes on the Brownian movement and Tyndall effect.
Answer:
1. Brownian movement:
- zig-zag movement of colloidal particles.
2. Tyndal effect:
- Scattering of light by colloidal particles which illuminates the path of beam in the colloidal dispersion.
Question 9.
Gas mask is used in coal mines. Why? What is the principle behind it?
Answer:
In order to adsorb poisonous gases. The principle involved is adsorption.
Plus Two Chemistry Surface Chemistry Three Mark Questions and Answers
Question 1.
Colloids can be converted into precipitates on the addition of electrolytes.
- Name the above phenomenon.
- What do you mean by coagulating value?
Answer:
- Coagulation.
- The minimum concentration of an electrolyte in millimoles per liter required to cause precipitation of a sol in two hours is called coagulating value.
Question 2.
Observe the given figure.
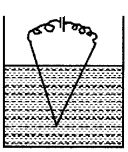
- Is there any mistake in the figure?
- If a high potential is applied between the upper ends of the wire what will be the result?
Answer:
- Yes, In this figure, the lower ends of the electrodes immersed in water are touching each other.
- If a high potential is applied between the upper ends of the wire, the circuit will be complete. There will be no colloid formation.
Question 3.
Name the method of preparation in which the following examples are involved.
- AS2O3 + 3H2S → AS2S3 + 3H2O
- SO2 + 2H2S → 2S + 2H2O
- FeCl3 + 3H2O → Fe(OH)3 + 3HCl
Answer:
- Double decomposition
- Oxidation
- Hydrolysis
Question 4.
Emulsions are liquid-liquid colloidal systems.
- What are the two types of emulsions?
- Explain the difference between milk and butter.
Answer:
- It is a colloidal form in which liquid is dispersed in liquid.
- Milk is an oil in water type emulsion and butter is a water in oil type emulsion.
Question 5.
In a debate a student argued that “Colloid is neither a true solution nor a suspension.”
- Do you agree with this argument?
- List the properties of colloids.
Answer:
1. Yes
2. properties of colloids:
- Colligative properties
- Mechanical properties
- Optical properties
- Electrical properties
Question 6.
Match the following:
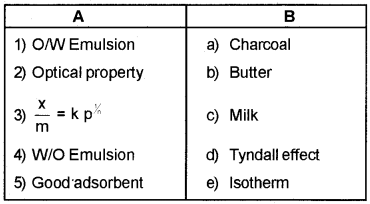
Answer:
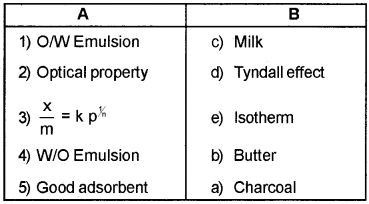
Question 7.
Complete the following:
- Lyophilic sol – starch; Lyophobic sol …………..
- Deltas are formed where rivers and seawater meets. Do you agree with this statement? Explain.
Answer:
- Lyophobic sol – gold sol (or any other correct example).
- Yes. River water is a colloid of clay. Seawater contains a number of electrolytes. When river water meets the seawater, the electrolytes present in seawater coagulate the colloid of clay resulting in its deposition with the formation of delta.
Question 8.
- Arrange the following electrolytes in the increasing order of coagulating power for the coagulation of Fe(OH)3sol.
Na3(PO4)2, NaCl, K4[Fe(CN)6], CaSO4
- Name the underlying principle.
Answer:
- NaCl < CaSO4 < Na3(PO4) < K4[Fe(CN)6.
- Hardy-Schulze rule.
Question 9.
What is the difference between multimolecular and macromolecular colloids? Give one example of each. How are associated colloids differ from these two types of colloids?
Answer:
Multimolecular colloids are formed by the aggregation of a large number of small atoms/molecules. The aggregates thus formed have size in the colloidal range, e.g. Gold sol.
Macromolecular colloids contain large size molecules which have the dimensions of colloids, e.g. Starch. Associated colloids behave as electrolytes at low concentration but exhibit colloidal behaviour at higher concentration, e.g. Soap.
Question 10.
Explain what is observed
- When a beam of light is passed through a colloidal sol?
- An electrolyte, NaCl is added to hydrated ferric oxide sol?
- Electric current is passed through a colloidal sol?
Answer:
- Scattering of light by the colloidal particles takes place and the path of light become illuminated. This is called Tyndall effect.
- The positively charged colloidal particles of Fe(OH)3 sol get coagulated by the oppositely charged ion provided by the electrolyte.
- On passing direct current, colloidal particles move towards the oppositely charged electrode where they lose their charge and get coagulated.
Plus Two Chemistry Surface Chemistry Four Mark Questions and Answers
Question 1.
A graph wrongly plotted between temperature and rate of adsorption for chemical adsorption is given below:
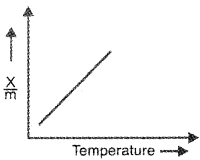
a. Draw it correctly.
b. Draw the graph of rate of adsorption and temperature for physical process.
c. Write three or four factors influencing the adsorption of a gas on a solid.
Answer:
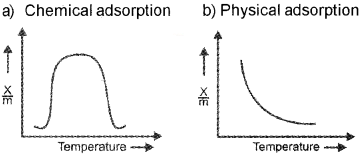
c. Three or four factors influencing the adsorption of a gas on a solid
- Nature of adsorbent
- Nature of adsorbate
- Temperature
- Pressure
Question 2.
Gold sol is to be prepared from rods of gold.
- Which method do you suggest?
- Explain.
- Distinguish between sol and gel with suitable examples for each.
Answer:
1. Electrical disintegration method – Bredig’s Arc Method.
2. An electric arc is struck between electrodes of the metal immersed in dispersion medium. The heat produced vapourises the metal which then condenses to form colloids.
3. Difference between sol and gel with suitable examples:
- Sol – Colloids in which dispersed phase is solid and dispersion medium is liquid, e.g. Paints, Cell fluids.
- Gel – Colloids in which dispersed phase is liquid and dispersion medium is solid, e.g. Cheese, Butter.
Question 3.
For a question, ‘What is emulsion? a student wrote ‘It is a colloidal form in which a liquid is dispersed in solid.
- Is it correct?
- What do you mean by emulsion?
- How can we increase the stability of an emulsion?
Answer:
- No.
- It is a colloidal form in which a liquid is dispersed in another liquid.
- Stability of emulsion can be increased by using emulsifying agents (certain substances like proteins, gums, soaps, detergents, lampblack, etc.)
Question 4.
Analyse the following statements:
Statement 1: Negatively charged Arsenic sulphide can be effectively precipitated using Al3+ ion than Na+ ion.
Statement 2: There is no difference in the precipitation of arsenic sulphide by Al3+ ion and Na+ ion.
- Which statement is correct? Justify.
- Name the law behind your opinion.
- State the law.
Answer:
- Statement 1. Precipitating power of an ion is directly proportional to its valency.
- Hardy-Schulze rule.
- A colloid can be precipitated by an ion having opposite charge to that of the colloid. Greaterthe valency of coagulating ion added greater its power to precipitate.
eg: AI3+ > Mg2+ > SO42- > Na+ > PO3+ > Cl–
Question 5.
What is observed in the following situations?
- When a beam of light is passed through a colloid.
- When an electrolyte, NaCl is added to hydrated ferric hydroxide sol.
Answer:
- Tyndall cone is observed due to Tyndall effect. Scattering of light by colloidal particles is called Tyndall effect.
- The coagulation of ferric hydroxide sol is observed. This is in accordance with Hardy-Schulze rule.
Question 6.
Adsorption is a surface phenomenon.
- Give an example for adsorption.
- Name the following plot. What do x/m and Ps stands for?
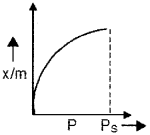
Answer:
- Adsorption of Cl2 on charcoal.
- Freundlich adsorption isotherm, x/m represents rate of adsorption (‘x’ is the mass of the gas adsorbed on mass ‘m’ of the adsorbent) and Ps is the pressure at which maximum adsorption takes place (saturation pressure).
Question 7.
Explain the following terms:
- Peptization
- Electrophoresis
Answer:
- Peptization – It is the process of converting a precipitate into a colloidal solution.
- Electrophoresis – The migration of colloidal particles, under the influence of an electric field is called electrophoresis.
Question 8.
- Define the terms ‘Kraft temperature’ and ‘Critical micelle concentration’.
- Explain the cleansing action of soap.
Answer:
1. Kraft temperature (Tk) – The temperature above which micelle formation occurs. Critical micelle concentration (CMC) – The concentration above which micelle formation occurs.
2. Soap is sodium or potassium salt of higher fatty acids. At a particular concentration the soap molecules will rearrange in such a way that its polar end is towards water and non-polar end towards oil.
These molecules aggregate to form ionic micelles around the oil/dirt particles. Since the polar groups can interact with water, the micelles are pulled in water and are removed from the dirty surface. Thus soap helps in emulsification and washing away of oils and fats.
Question 9.
Colloid of gold is prepared by striking an electric arc between gold electrodes kept in water.
- What is this method known as? Why is it regarded as a dispersion method?
- What is the charge of gold sol? How do particles acquire this charge?
Answer:
1. Bredig’s arc method. This process involves dispersion as well as condensation. When an electric arc is struck between the metal electrodes immersed in the dispersion medium, due to the intense heat produced the metal gets dispersed by vapourisation which then condenses to form particles of colloidal size.
2. Negative change. Due to electron capture by gold sol particles during electrodispersion of gold.
Question 10.
When excess of an electrolyte is added, the colloidal particles are precipitated.
- Give the reason for precipitation.
- Arrange the following ions in the increasing order of the flocculating power in coagulation of negative sol. K+, Ca2+, Fe3+
Answer:
1. A colloid can be precipitated by an ion having opposite charge to that of the colloid. Greater the valency of coagulating ion added greater its power to precipitate.
eg: Al3+ > Mg2+ > SO42- > Na+ > PO3+ > Cl–
2. K+ < Ca2+ < Fe3+
3. These are arranged based on Hardy-Shulze rule.
Question 11.
Many food items are colloids in one form or the other.
- To which category of colloids does cheese belongs?
- Explain how sols are classified depending upon the nature of the interaction between the dispersed phase and dispersion medium. Give suitable examples.
Answer:
- Cheese belongs to gel (Liquid in solid type colloid).
- Based on the nature of interaction between dispersed phase and dispersion medium sols are divided into two categories:
a. Lyophilic colloids:
These are solvent-loving colloidal sols which are directly formed by mixing the dispersed phase with the dispersion medium. These are reversible, quite stable and cannot be easily coagulated, e.g. gum, gelatine, starch, rubber.
b. Lyophobic colloids:
These are solvent-hating sols which can be prepared only by special methods. These are irreversible, not stable and are easily coagulated on addition of small amounts of electrolytes. e.g. sols of metals and their sulphides.
Question 12.
Anhydrous CaCl2 and silica gel differ in their behaviour towards moisture.
- Identify the phenomenon taking place in both cases.
- How is adsorption affected with pressure? Explain.
Answer:
1. Anhydrous CaCl2 absorbs moisture. The phenomenon is absorption. Silica gel absorbs moisture. The phenomenon is adsorption.
2. The rate of adsorption increases with increase in pressure. Freudlich’s adsorption expression is given by
as = kp1/n (n > 1)
The graphical representation is,
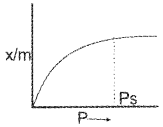
At high pressure rate of adsorption attains a maximum value and after that pressure is having no influence. At low pressure, rate of adsorption is directly proportional to the pressure.
Question 13.
Gelatin in icecream prevents the formation of large crystals of ice.
- What is the role of gelatin here?
- Justify the property of gelatin here as lyophilic colloid.
Answer:
1. Gelatin acts as a protective colloid.
2. Gelatin is a lyophilic (liquid loving) colloid and can act as a protective colloid. The colloid can be formed by simply mixing gelatin with ice cream. Also, it is very stable and difficult to get coagulated. Here gelatin forms a layer around the particles of ice cream and prevent the later from forming large crystals.
Question 14.
Milk is a colloid.
- Do you agree with this statement?
- What is the difference between dispersed phase and dispersion medium?
- What do you mean by lyophilic colloid?
Answer:
- Yes.
- The medium in which dispersion takes place is called dispersion medium and the substance which undergo dispersion is called dispersed phase.
- If there is strong attractive force between the dispersed phase and dispersed medium that colloid is called lyophilic colloid.
Question 15.
Many industrial processes use catalysts.
- What are catalysts?
- What do you mean by the terms ‘promoters’ and, ‘poisons’ in catalysis?
- Suggest an example for a catalytic promoter.
Answer:
- Catalysts are substances, which alter the rate of a chemical reaction and themselves remain unchanged.
- Promoters are substances that enhance the activity of a catalyst. Catalytic poisons are substances which decrease the activity of a catalyst.
- In Haber’s process for manufacture of ammonia, molybdenum (Mo) acts as a promoter for iron catalyst.
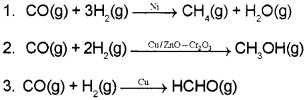
![]()
Question 16.
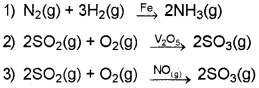
- Classify the above reactions into Homogeneous and Heterogeneous catalysis.
- What is shape-selective catalysis?
Answer:
- (1) and (2) are heterogeneous catalysis and (3) is homogeneous catalysis.
- The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape-selective catalysis.
Zeolites are good shape-selective catalysts because of their honeycomb-like structure. The reactions taking place in zeolites depend upon the size and shape of reactant and product molecules as well as upon the pores and cavities of the zeolites.
Question 17.
You are supplied with the following chemicals NaCl, BaCl2, AlCl3.
- Which of these would you prefer most to coagulate a sample of As2S3 sol?
- Arrange the following electrolytes in the increasing order of coagulating power.
NaCl, BaCl2, AlCl3 - Name and state the rule based on which you have arrived at the coagulating power of these substances.
Answer:
- AlCl3
- NaCl < BaCl2 < AlCl3
- Hardy – Schulze rule. It states that greater the valence of the flocculating ion greater is its power to cause precipitation.
Plus Two Chemistry Surface Chemistry NCERT Questions and Answers
Question 1.
Give reason why a finely divided substance is more effective as an adsorbent.
Answer:
Adsorption is a surface phenomenon. Since finely divided substance has large surface area, hence, adsorption occurs to a greater extent.
Question 2.
What do you understand by activation of adsorbent? How is it achieved?
Answer:
Activation of adsorbent implies increase in the adsorption power of the adsorbent. It involves increase in the surface area of the adsorbent and is achieved by the following methods:
- by finely dividing the adsorbent into small grains.
- by removing the gases already adsorbed.
- by making the surface of adsorbent rough by chemical or mechanical methods.
Question 3.
What role does adsorption play in heterogeneous catalysis?
Answer:
In heterogeneous catalysis, the reactants are generally gases whereas catalyst is a solid. The reactant molecules are adsorbed on the surface of the solid catalyst by physisorption or chemisorption. As a result, the concentration of the reactant molecules on the surface increases and hence the rate of reaction increases.
Alternatively, one of the reactant molecules undergoes fragmentation on the surface of the solid catalyst producing active species which react faster. The product molecules, in either case, have no affinity for the solid catalyst and are desorbed making the surface free for fresh adsorption. This theory is called adsorption theory.
Question 4.
What is the difference between multimolecular and macromolecular colloids? Give one example of each. How are associated colloids different from these two types of colloids?
Answer:
Multimolecular colloids are formed by the aggregation of a large number of small atoms/molecules. The aggregates thus formed have size in the colloidal range, e.g. Gold sol.
Macromolecular colloids contain large size molecules which have the dimensions of colloids, e.g. Starch. Associated colloids are formed by surface active molecules having polar as well as non-polar ends.
They behave as electrolytes at low concentration but beyound critical micelle concentration and above the Kraft temperature, they associate together to form ionic micelles whose size lies in the colloidal range, e.g. Soap.
Question 5.
Explain what is observed
- When a beam of light is passed through a colloidal sol?
- An electrolyte, NaCl is added to hydrated ferric oxide sol?
- Electric current is passed through a colloidal sol?
Answer:
- Scattering of light by the colloidal particles takes place and the path of light become illuminated. This is called Tyndall effect.
- The positively charged colloidal particles of Fe(OH)3 sol get coagulated by the oppositely charged ion provided by the electrolyte.
- On passing direct current, colloidal particles move towards the oppositely charged electrode where they lose their charge and get coagulated.
We hope the given Plus Two Chemistry Chapter Wise Questions and Answers Chapter 5 Surface Chemistry will help you. If you have any query regarding Plus Two Chemistry Chapter Wise Questions and Answers Chapter 5 Surface Chemistry, drop a comment below and we will get back to you at the earliest.
