Plus Two Chemistry Chapter Wise Questions and Answers Chapter 1 The Solid State are part of Plus Two Chemistry Chapter Wise Questions and Answers. Here we have given Plus Two Chemistry Chapter Wise Questions and Answers Chapter 1 The Solid State.
| Board | SCERT, Kerala |
| Text Book | NCERT Based |
| Class | Plus Two |
| Subject | Chemistry Chapter Wise Questions |
| Chapter | Chapter 1 |
| Chapter Name | The Solid State |
| Number of Questions Solved | 60 |
| Category | Kerala Plus Two |
Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 1 The Solid State
Plus Two Chemistry The Solid State One Mark Questions and Answers
Question 1.
The 14 possible three dimensional crystal lattices are called ____________.
Answer:
Bravais Lattices
Question 2.
Which of the following type of cubic lattices has maximum number of atoms per unit cell?
(a) simple cubic
(b) body centred cubic
(c) face centred cubic
(d) all have same
Answer:
(c) face centred cubic (4 atoms per unit cell)
Question 3.
F- centres in an ionic crystal are
(a) lattice sites containing electrones
(b) interstitial sites containing electrons
(c) lattice cites that are vacant
(d) interstitial sites containing electrones
Answer:
(a) lattice sites containing electrons
Question 4.
The colour imparted by excess potassium in KCl crystal is ________.
Answer:
Violet (or lilac)
Question 5.
Which of the following substances show anti-ferromagnetism?
(a) ZrO2
(b) CdO
(c) CrO2
(d) Mn2O3
Answer:
(d) Mn2O3
Question 6.
The number of tetrahedral and octahedral voids in a ccp array of 100 atoms are respectively.
Answer:
200 and 100
Question 7.
Potassium dichromate belongs to which crystal system.
Answer:
Triclinic
Question 8.
A solid compound contains XYZ atoms in a cubic lattice with X atoms occupying the corners, Y atoms in the body centred positions and Z atoms at the centres of faces of the unit cell. What is the empirical formula of the compound Answer:
XYZ3
Question 9.
The empty space in body centred cubic lattice is
Answer:
32%
Question 10.
Which solid has the weakest intermolecular force
Answer:
ice
Question 11.
In crystalline solid, atoms, ions or molecules are held in an orderly array. But some point defect is observed in a crystal, when a vacancy is created by an atom or ion dislocated from its normal position to an interstitial site. What is the defect called?
Answer:
Frenkel defect
Plus Two Chemistry The Solid State Two Mark Questions and Answers
Question 1.
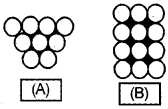
- Write the names of A and B?
- Identify and write the name of the shaded parts of A and B?
Answer:
1. Names of A and B are:
- Hexagonal close packing.
- Square close packing,
2. shaded parts of A and B are:
- Is octahedral void; in
- Is tetrahedral void.
Question 2.
Teacher said that Frenkel defect will not happen in alkali metal halides. Ramu asked the reason for this. Can you explain?
Answer:
Frenkel defect is favoured by the small size of cations. In alkali metal halides both cations and anions are of almost same size.
Question 3.
“Dielectric substances are related to conductors.”
“Dielectric substances do not conduct electricity at normal condition”
These are two arguments of a class discussion.
- Do you agree with these arguments?
- If yes, justify both statements?
Answer:
- Yes.
- Dielectric substances do not conduct electricity through them. But they can be made conductors either by heating the substances or by applying mechanical stress.
Question 4.
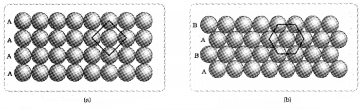
- Identify A and B.
- Explain them.
Answer:
1. A and B.
- A – Square close packed (scp) arrangement in two dimensions.
- B- Hexagonal close packed (hep) arrangement in two dimensions.
2. Explanation:
(a) A – Square close packed:
(scp) arrangement in two dimensions – particles of the second row are arranged just below the first row. Similarly, particles of third row are arranged just below the particles of the second row and so on. Here vertical as well as horizontal alignment is possible. In this arrangement each particle is in direct contact with four other particles and the coordination number is 4.
(b) B- Hexagonal close packed:
(hep) arrangement in two dimensions – In this arrangement, particles of second row are arranged in the depressions of the particles of the first row. Similarly the particles of the third row are arranged in the depressions of the particles of the second row. In this arrangement, each particle is in direct contact with six other particles and the coordination number is 6.
Question 5.
- Name the unit of magnetic moment.
- Match the following.
- Paramagnetic – Fe3O4
- Ferromagnetic – O2
- Antiferromagnetic – CrO2
- Ferrimagnetic – MnO
Answer:
- Bohr magneton (μB)
- Match
- Paramagnetic – O2
- Ferromagnetic – CrO2
- Antiferromagnetic – MnO
- Ferrimagnetic – Fe3O4
Question 6.
All crystals exhibit imperfections.
- Which law is related to this statement?
- Draw the picture showing the Frenkel defect.
Answer:
1. Third law of thermodynamics.
2.
Question 7.
What do you mean by Anisotropy how is it differ from isotropy
Answer:
| Anisotropic | Isotropic |
| Physical properties have different values along different directions | Physical property would be same in any direction |
| Because they have different arrangement in different directions | Because arrangement is irregular in any direction |
Question 8.
A cubic solid is made of two elements P and Q. Atoms of Q are at the corners of the cube and P at the body-centre. What is the formula of the compound? What are the coordination numbers of P and Q?
Answer:
The atom at the corner makes 18 contribution while atom at body centre makes 1 contribution to the unit cell.
No. of atoms of Q per unit cell = 8 × 18 = 1
No. of atoms of P per unit cell = 1 × 1 = 1 Therefore, formula of the compound is PQ.
The atom at the body centre would be in contact with all the atoms at the corners. Hence, the coordination number of P is 8.
Similarly, coordination number of Q is 8 because it is shared by 8 other atoms.
Question 9.
Packing efficiency differ for B.C.C, F.C.C and Simple cube what is packing efficiency?
Answer:
Packing efficiency is the % of total space filled by the particle.
OR
Packing efficiency = \(\frac{\text { Volume occupied by spheres in the unit cell }}{\text { Total volume of the unit cell }} \times 100[/latex ]
Question 10.
If NaCl is doped with 10-3 mol % of SrCl2, what is the concentration of cation vacancies?
Answer:
Every Sr2+ ion causes one cation vacancy (because two Na+ ions are replaced by one Sr2+ and it occupies the site of one Na+ ion and the other site remains vacant.) Therefore, introduction of 10-3 moles of SrCl2 per 100 moles of NaCl would introduce 10-3 moles cation vacancies in 100 moles of NaCl.
No. of vacancies per mole of NaCl = [latex]\frac{10^{-3}}{100}\) × 6.02 × 1023 = 6.02 × 1018
Question 11.
Even crystal, the substance which we consider as the most perfect solid, shows some defects or imperfections.
- Which law in thermodynamics deals with this topic?
- Explain the law.
Answer:
- Third law of thermodynamics
- Entropy of a perfectly crystalline substance is zero at absolute zero temperature.
Question 12.
Excess of Li makes LiCl crystal pink and excess of K makes KCl crystals lilac. Is this true? How will you account for the above processes?
Answer:
Yes. This is an example for metal excess defect due to anion vacancies. The colour is due to the presence of F – centre in the crystals.
Question 13.
What are the consequences of Schottky and Frenkel defects?
Answer:
Frenkel defect does not change the density of the crystal but the Schottky defect decreases the density of the substance.
Question 14.
Calculate the number of atoms per unit cell of silver which crystallizes in fee lattice.
Answer:
- Contribution of particles from one corner = 1/8
- Contribution from 8 corners = 1/8 × 8 = 1
- Contribution from 6 face centres = 1/2 × 6 = 3
- Total number of particles present in the unit cell of the crystal = 1 + 3 = 4
- Number of atoms present in one unit cell of Ag = 4.
Question 15.
Explain the type of voids found in three-dimensional close packing in crystals.
Answer:
Two voids
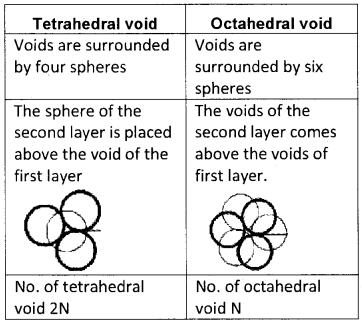
Plus Two Chemistry The Solid State Three Mark Questions and Answers
Question 1.
In an answer paper a student wrote as “carborundum crystals are very soft.”
- Do you agree with this?
- What is your opinion?
- In which crystal type carborundum is included?
- Substantiate your view.
Answer:
- No.
- Carborundum crystals are very hard.
- Covalent crystal.
- In the case of covalent crystals the constituent particles are atoms and they are held together by strong covalent bond network. In carborundum covalent bond network is constituted by Si and C atom which are held very strongly at their positions. Hence, carborundum is a covalent crystal and is very hard.
Question 2.
During a seminar a student asked another student, “Can NaCl give flame test?”
- Write your answer.
- Write the colour of sodium during flame test.
- Write the name of the point which is responsible for the colour of alkali metal halides having excess metal ions.
Answer:
- Yes
- Golden yellow
- F-centre electron trapped anion vacancy
Question 3.
Solids are classified into two types.
- What are they?
- Give two examples.
- Give 3 features of them.
Answer:
1. Crystalline and amorphous.
2. Crystalline – NaCl, KCl. Amorphous-Plastic, rubber.
3.
| Crystalline Solids | Amorphous Solids |
| 1. Sharp melting point | 1. Melting point is in a range of temperature |
| 2. Long range order | 2. Short range order |
| 3. Anisotropic | 3. Isotropic |
| 4. Definite shape | 4. Irregular shape |
Question 4.
Teacher explained that due to a stoichiometric defect, the density of a crystal changes.
- Name the defect.
- What change can we observe?
- Give an example.
Answer:
- Schottky defect
- Density decrease
- NaCl
Question 5.
1. Packing efficiency is the percentage of total space filled by the particles. Which of the following lattices has the highest packing efficiency? Simple cubic lattice, body centered cubic lattice, hexagonal close packed lattice.
2. An element has a body centred cubic structure with a cell edge of 288 pm. The density of the element is 7.2 g/cm3. How many atoms are present in 208 g of the element?
Answer:
1. Hexagonal close packed lattice.
2. a = 288 pm = 288 × 10-10 cm
Volume of the unit cell = (288 × 10-10cm)3
= 23.9 × 10-24cm3
Volume of 208 g of the element
208g7.2gcm−3 = 28.88 cm3
∴ Number of units cells in 208 g =
28.88cm323.9×10−24cm3 = 1.208 × 1024
For bcc structure, number of atoms in one unit cell = 2
∴ Number of atoms in 208 g = 2 × 1.208 × 1024
= 2.416 × 1024
Question 6.
Derive packing efficiency of
- ccp and hep structure
- Body centered cubic
- Simple cubic
Answer:
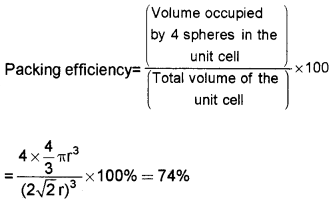
1. Packing Efficiency in ccp and hep Structures:
In the case of ccp and hep, the edge length
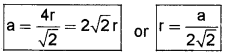
We know that each unit cell in ccp structure has 4 spheres.
Volume of sphere = 4πr33
Volume of the cube = a3
2. Packing Efficiency of Body Centred Cubic Structures:
In this case radius of a sphere.
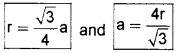
We know that bcc has 2 spheres in the unit cell.
∴ Packing efficiency = 2×43πr3[(4√3r)]3 × 100% = 68%
3. Packing Efficiency is Simple Cubic Lattice:
In simple cubic lattice edge length ‘a’ and radius of the sphere ‘r’ are related as,
![]()
We know that a simple cubic unit cell contains only one sphere.
∴ Packing efficiency = 1×43πr3(2r)3 × 100%
= 52.36%
= 52.4%
Question 7.
Iron (II) oxide crystallise in cubic structure with unit cell edge of 5.0 Å If the density of the oxide is 3.8 g cm-3. Calculate the no.of Fe2+ and O2- present in each unit cell, [atomic mass of Fe = 56, O = 16] (3)
Answer:
Cell edge a = 5.0Å = 5.0 × 10-1 m = 5 × 10-8 cm
Density = 3.8 g/cm3 Molecular mass of FeO = 56 + 16 = 72 u
NA = 6.022 × 1023
d = ZMNAa3
Z = dNAa3M=3.8×6.022×102372×(5×10−8)3
= 3.97 = 4
Each cell contain 4 FeO molecule every FeO molecule contain one Fe2+ and one O2- ion. So no. of Fe2+ ion =4 no.of O2- ion = 4
Question 8.
Which are the two types of close packing in two dimension. What are its differences?
Answer:
1. Square close packing or AAA type.
- Coordination no.4. One sphere is in touch with 4 other spheres
- 2nd raw placed exactly under the first raw

2. Hexagonal close packing or AB AB Type.
- Coordination no.6 ie. one spheres is in touch with other six spheres.
- 2nd raw is placed in the depressions of first raw.
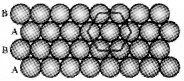
Question 9.
Classify the following solids as ionic, metallic, molecular, network (covalent) or amorphous.
- Tetraphosphorus decoxide (P4O10)
- Ammonium phosphate (NH4PO4)
- SiC
- I2
- P4
- Plastic
- Graphite
- Brass
- Rb
- LiBr
- Si
Answer:
- Tetraphosphorus decoxide (P4O10) – Molecularsolid
- Ammonium phosphate (NH4PO4) – Ionic solid
- SiC – Network(covalent) solid
- I2 – Molecular solid
- P4 – Molecular solid
- Plastic-Amorphous solid
- Graphite – Network(covalent) solid
- Brass – Metallic solid
- Rb- Metallic solid
- LiBr – Ionic solid
- Si – Network(covalent) solid
Question 10.
A substance A’ crystallizes in fee lattice.
- Calculate the number of atoms present per unit cell of ‘A’.
- In a crystalline solid AB, some vacancy is produced by missing of equal number of oppositely charged ions. What is the defect called?
Answer:
- The number of atoms present per unit cell of ‘A’.
- Contribution from 8 corners = 1/8 × 8 = 1
- Contribution from centres of 6 faces = 1/2 × 6 = 3
- Total number of atoms of A’ per unit cell = 1 +3 = 4
- Schottky defect
Question 11.
- What is meant by the term coordination number?
- What is the coordination number of atoms:
- In a cubic close-packed lattice?
- In a body-centred cubic structure?
Answer:
- The number of nearest neighbours in a packing is called coordination number.
- The coordination number of atoms
- In cubic-close packed structure each atom is in direct contact with 12 other atoms. Hence, its coordination number is 12.
- In a body centred cubic structure coordination number is 8
Question 12.
Identify the crystal.
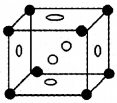
- Write the name of the crystal.
- How many particles are present the unit cell of this crystal?
- Write the relation connecting edge length and radius of the particle.
Answer:
- Face centred cubic crystal.
- Particles are present the unit cell of this crystal.
- Contribution from 8 corners = 1/8 × 8 = 1
- Contribution from 6 face centers = 1/2 × 6 = 3
- The total number of particles = 4
- r = √2a4=a2√2.
Question 13.
1. The following diagram shows the alignment of magnetic moments for some magnetic properties.
- ↑↑↑↑↑↑
- ↑↓↑↓↑↓
- ↑↑↓↑↑↓
Identify the magnetic properties denoted by (a), (b), (c).
2. Examine the substances H2O, NaCl, C6H6 and name the magnetic property common to them.
Answer:
1. The alignment of magnetic moments for some magnetic properties.
- Ferromagnetic
- Antiferromagnetic
- Ferrimagnetic
2. Diamagnetism
Question 14.
How many lattice points are there in one unit cell of the following lattices?
- Face centred cubic
- Body centred cubic
- Simple cubic
Answer:
- Face centred cubic – 4
- Body centred cubic – 2
- Simple cubic – 1
Plus Two Chemistry The Solid State Four Mark Questions and Answers
Question 1.
- Classify the following into crystalline and amorphous solids.
- NaCl
- Graphite
- Plastic
- Diamond
- Rubber
- KCl
- Wood
- CaCO3
- Iodine
- Write any two properties of graphite.
Answer:
| Crystalline solids | Amorphous solids |
| NaCl, Graphite | Plastic |
| Diamond, CsCl | Rubber |
| CaCO3, Iodine | Wood |
- Two properties of graphite
- It is a good conductor of electricity.
- Graphite is a good solid lubricant.
Question 2.
Fill in the blanks.

Answer:

Question 3.
- NaCl shows mainly Schottky defect and AgCl shows Frenkel defect. Do you agree with this statement? Justify.
- Classify the following solids into isotropic and anisotropic.
Polyvinylchloride, Rubber, Glucose, Glass
Answer:
- Yes. In NaCl, both Na+ and Cl– are of almost same size and hence it shows Schottky defect. But in AgCl, Ag+ is smaller than Cf. Hence, it shows Frenkel defect.
- Anisotropic-Glucose
Isotropic – Glass, Rubber, Polyvinylchloride
Question 4.
The common salt, sodium chloride is an example for a crystal system with edge length a = b = c and axial angles α = β = γ = 90°.
- Identify the crystal system.
- What happens when sodium chloride crystal is heated in presence of sodium?
Answer:
- Cubic crystal
- Gives yellow colour to the crystal due to F centre, ie. electron trapped anion vaccancy. It is a metal excess defect.
Question 5.
Stoichiometric defects are of two types such as vacancy defects and interstitial defects.
- Which defect is basically a vacancy defect in ionic solids?
- Which stoichiometric defect causes a decrease in the density of solid?
- On heating white ZnO it turns yellow. Which is the crystal involved here? Explain.
Answer:
- Schottky defect
- Schottky defect
- It is due to metal excess defect caused by the presence of extra cations at interstitial sites. ZnO is white in colour at room temperature. On heating it loses oxygen and turns yellow.
![]()
The excess Zn2+ ions move to interstitial sites and the electrons to neighbouring interstitial sites. The yellow colour of non-stoichiometric ZnO (Zn1+xO) is due to these trapped electrons.
Question 6.
- Why is Frenkel defect not found in alkali metal halides?
- A crystalline solid has simple cubic structure in which P atoms are present at the corners, Q atoms are present at the edge centres and R atoms are present at the centre of the unit cell. What is the formula of the compound?
Answer:
1. Frenkel defect is favoured by the small size of cations. In alkali metal halides both cations and anions are of almost same size.
2. Number of atoms of P = 18 × 8 = 1
Number of atoms of Q = 14 × 12 = 3 4
Number of atoms of R = 1
∴ Formula of the compound is PQ3R
Question 7.
- Classify each of the following solids as ionic, metallic, molecular, network or amorphous.
- I2
- Plastic
- LiBr
- SiC
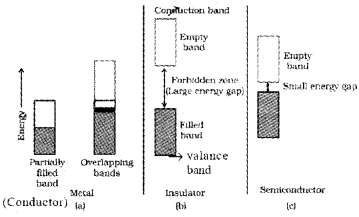
- In terms of band theory differentiate Conductors, insulators & semi conductors
Answer:
- Classification of solids as ionic, metallic, molecular, network or amorphous.
- I2 – Molecular
- Plastic – Amorphous
- LiBr – Ionic
- SiC – Covalent
- Differences between Conductors, insulators & semi conductors.
- Conductors:
The valence band overlaps with the conduction band or no energy gap exists between them. - Insulators:
The energy gap between valance band and conduction band is very large. Hence electrons from valence band cannot move into the conduction band. Semi conductors have small energy gap between valence band and conduction band.
- Conductors:
Question 8.
On the basis of nature of constituent particles crystals are classified into four types.
- Which are they?
- In which type does diamond belongs to? Why?
- Can you say whether Iodine can be written as an example of ionic crystal? Why?
Answer:
- Ionic crystals, Molecular crystals, Covalent crystals and Metallic crystals.
- Diamond belongs to covalent crystals, because in diamond, the constituent particles are carbon atoms connected by strong covalent bonds.
- No. In ionic crystal the constituent particles are ions. Since the constituent particles of iodine are molecules, it is a molecular crystal.
Question 9.
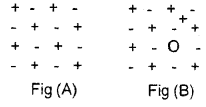
- Distinguish between A and B?
- Explain the defect in figure B.
- Give two examples of crystals showing this defect.
Answer:
- Fig. A – Ideal crystal. Fig. B – Frenkel defect.
- Frenkel defect is due to the migration of cation from its original position to the void. This type of defect is favoured by the small size of cation. As a result of this defect, the neutrality, density and stoichiometry remain the same. But the conductivity increases.
- AgCl, AgBr
Question 10.
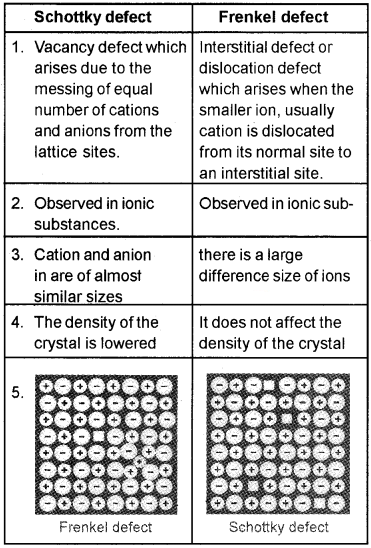
The teacher explained crystal defects in classroom.
- What are the different types of crystal defects?
- Explain with the help of diagram the important difference between Schottky and Frenkel defects?
Answer:
1. Stoichiometric defects and Non-Stoichiometric defects.
2.
Question 11.
NaCl is an example for diamagnetic substance.
- Write an example for paramagnetic substance.
- What is the difference between ferromagnetic and anti ferromagnetic substances?
- In case of ferri magnetic substances net magnetic moment is not zero. Justify.
Answer:
1. CuO
2. & 3.
Question 12.
The diagram of a cubic crystal whose molecular mass = M, edge length = a, density = ρ, is given below. N is the Avogadro number.
- From the above given details find the mass of this cube and also the mass of N particles?
- By equating these two equations, try to find out a suitable equation for the density of this cube.
Answer:
1. Mass of cube = volume × density = a3 ρ ……………. (1)
Mass of N particles = N × Mass of one particle
= N × MNA (M = Gram Atomic Mass) …………. (2)
2. The equations (1) & (2) represent the mass of the cube.
i.e., a3p = N × MNA
∴ ρ=N×Ma3×NA
Question 13.
- Classify each of the following as being either a p- type or a n-type semiconductor.
- Ge dopped with In
- Si dopped with B
- A compound is formed by two elements P and Q. The element Q forms ccp and atoms of P occupy 1/3rd of the tetrahedral voids. What is the formula of the compound?
Answer:
1. Either a p- type or a n-type semiconductor
- Ge dopped with In → p-type
- Si dopped with B → p-type
2. The formula of the compound
Q = 4
No. of tetrahedral voids = 2N = 2 × 4 = 8
P = 1/3 × 8 = 8/3
Q8/3P4 = Q8P12 = Q2P3
Plus Two Chemistry The Solid State NCERT Questions and Answers
Question 1.
Classify the following solids as ionic, metallic, molecular, network (covalent) or amorphous.
- Tetraphosphorus decoxide (P4O10)
- Ammonium phosphate (NH4PO4)
- SiC
- I2
- P4
- Plastic
- Graphite
- Brass
- Rb
- LiBr
- Si
Answer:
- Tetraphosphorus decoxide (P4O10) – Molecularsolid
- Ammonium phosphate (NH4PO4) – Ionic solid
- SiC – Network(covalent) solid
- I2 – Molecular solid
- P4 – Molecular solid
- Plastic-Amorphous solid
- Graphite – Network(covalent) solid
- Brass – Metallic solid
- Rb- Metallic solid
- LiBr – Ionic solid
- Si – Network(covalent) solid
Question 2.
- What is meant by the term coordination number?
- What is the coordination number of atoms:
- In a cubic close-packed lattice?
- In a body-centred cubic structure?
Answer:
- The number of nearest neighbours in a packing is called coordination number.
- The coordination number of atoms
- In cubic-close packed structure each atom is in direct contact with 12 other atoms. Hence, its coordination number is 12.
- In a body centred cubic structure coordination number is 8
Question 3.
How many lattice points are there in one unit cell of each of the following lattice?
- Face-centred cubic
- End-centred monoclinic
- Body-centred
Answer:
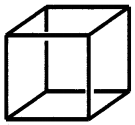
1. A face-centred cubic unit cell has lattice points at the corners of the cube and at face centres. There are eight comers and six faces of the cube. Each atom at corner makes a contribution of 18 while each atom at face centre makes a contribution of 12 to the unit cell.
Therefore, the number of atoms per unit cell
= 8 × 18 + 6 × 12 = 1 + 3 = 4
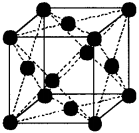
2. A end-centred monoclinic unit cell has lattice points at the face centres of only one set (two) of faces, in addition to the lattice points at the comers of the unit cell.
Therefore, the number of atoms per unit cell =
8 × 18 + 2 × 12 = 1 + 1 = 2
3. A body centred cubic unit cell has lattice points at the comers of the cube and at the body centre.
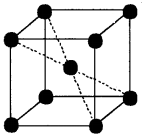
Therefore, the number of atoms per unit cell
= 8 × 18 + 1 × 1 = 1 + 1 = 2
Question 4.
A cubic solid is made of two elements P and Q. Atoms of Q are at the corners of the cube and P at the body-center. What is the formula of the compound? What are the coordination numbers of P and Q?
Answer:
The atom at the corner makes 18 contribution while atom at body centre makes 1 contribution to the unit cell.
No. of atoms of Q per unit cell = 8 × 18 = 1
No. of atoms of P per unit cell = 1 × 1 = 1 Therefore, formula of the compound is PQ.
The atom at the body centre would be in contact with all the atoms at the corners. Hence, the coordination number of P is 8.
Similarly, the coordination number of Q is 8 because it is shared by 8 other atoms.
Question 5.
If NaCl is doped with 10-3 mol % of SrCl2, what is the concentration of cation vacancies?
Answer:
Every Sr2+ ion causes one cation vacancy (because two Na+ ions are replaced by one Sr2+ and it occupies the site of one Na+ ion and the other site remains vacant.) Therefore, introduction of 10-3 moles of SrCl2 per 100 moles of NaCl would introduce 10-3 moles cation vacancies in 100 moles of NaCl.
No. of vacancies per mole of NaCl = 10−3100 × 6.02 × 1023 = 6.02 × 1018
We hope the given Plus Two Chemistry Chapter Wise Questions and Answers Chapter 1 The Solid State will help you. If you have any query regarding Plus Two Chemistry Chapter Wise Questions and Answers Chapter 1 The Solid State, drop a comment below and we will get back to you at the earliest.
