Plus Two Chemistry Chapter Wise Previous Questions Chapter 8 The d and f Block Elements is part of Kerala Plus Two Chemistry Chapter Wise Previous Year Questions and Answers. Here we have given Plus Two Chemistry Chapter Wise Questions and Answers Chapter 8 The d and f Block Elements.
Kerala Plus Two Chemistry Chapter Wise Previous Questions Chapter 8 The d and f Block Elements
Question 1.
a) Transition elements are ‘d’ block elements. (March – 2010)
i) Write any four characteristic properties of transition elements.
b) Lanthanoids and actinoids are f – block elements.
i) What is the common oxidation state of Lanthanoids?
ii) Name the Lanthanoid with common oxidation state +4.
iii) It is difficult to separate Lanthanoids in the pure state. Explain.
Answer:
a)
- They form coloured compounds.
- They exhibit variable oxidation state.
- They form complex.
- They are good catalysts,
b)
- + 3
- Cerium (Ce)
- Due to lanthanide contraction size is approxi mately equal. So separation is difficult.
Question 2.
Transition metals are widely used as catalysts in industrial processes. (Say – 2010)
a) Name any two industrial processes in which transition elements are used as catalysts.
b) Transition metals exhibit catalytic properties. Why?
c) Why do the transition elements exhibit greater similarity in properties compared to main group elements along the period as well as down the group?
Answer:
a) Fe – Haber’s process forthe manufacture of NH3 Ni – Hydrogenation of Oil for the manufacture of vanaspati ghee
b) Because of
- variable oxidation state of metals
- ability to form complexes
c) The outer electronic configuration remains almost same and hence they show horizontal similarity.
Question 3.
a) Atomic sizes increase as we come down a group, but in 4th group of the Periodic Table Zr, Hf have almost the same atomic sizes. Why? (March – 2011)
b) E° (standard electrode potential) values generally become less negative as we move across a transition series, but E° values of Ni2+/Ni and Zn2+/Zn values are exceptions. Justify.
Answer:
a) This is due to Lanthanide contraction. It is the phenomenon of regular decrease in atomic size across the lanthanoid series.
b) Due to the stability of completely filled d10 configuration of Zn2+ its ΔiHΘ2 is less. This is responsible for the high negative value of E0z2+n/zn ( – 0.76 V).
Question 4.
Transition elements are d – block elements, with some exceptions. Usually they are paramagnetic. They show variable oxidation states. They and their compounds show the catalytic property. (Say – 2011)
a) Zn (Atomic number 30) is not a transition element, though it is a d – block element. Why?
b) Which is more paramagnetic Fe2+ or Fe3+? Why?
c) Why do transition elements show variable oxidation states?.
d) What is the reason for their catalytic property?
Answer:
a) A true transition element is one which has incompletely filled d – orbitals in its ground or in their common oxidation states. The zinc atom has completely filled d – orbitals.
b) Fe3+ is more paramagnetic.
The paramagnetic character of a transition metal ion depends on the number of unpaired d – electrons present in it. Fe3+ wich 3d5 configuration has 5 unpaired d – electrons while Fe2+ with 3d6 configuration has only 4 unpaired d – electrons.
c) In transition elements the energy difference between (n – 1)d and ns orbitals is very less. Hence, along with ns electrons (n – 1)d electrons can also take part in chemical reactions.
d) Due to their ability to adopt multiple oxidation states and to form complexes.
Question 1.
a) Potassium dichromate (K2 Cr2 O7) is an important compound of chromium. Describe the method of preparation of potassium dichromate from chromite ore. (March – 2012)
b) The gradual decrease in the size of lanthanoid elements from lanthanum to lutetium is known as lanthanoid contraction. Write anyone consequence of lanthanoid contraction.
Answer:
a) K2 Cr2 O7 is prepared from chromate one Fe Cr2 O4.
Step I: The powdered ore is heated with molten alkali in free access of air to form soluble sodium chromate.
4 Fe Cr2 O4 + 16 NaOH + 7O2 → 8Na2CrO4 + 2Fe2O3 + 8H2O
Step II: Sodium chromate (Na2Cr O4) is filtered and acidified with dil. H2SO4 to form sodium dichromate.
2Na2CnO4 + H2SO4 → Na2Cn2O7 + Na2SO4 + H2O
Step III: Na2Cn2O7 solution is treated with KCI to form K2Cr2O7.
Na2Cn2O7 + 2 KCI → K2Cn2O7 + 2NaCI
b) Consequences of lanthanoid contraction ane
- Difficulty in separation of lanthanoids due to similanity in chemical properties.
- The similarity in size of elements belonging to same group of second & third transition series.
Question 1.
Assume that you are going to present a seminar on transition elements. Prepare a seminar paper by stressing any four important properties of transition elements. (Say – 2012)
Answer:
The transition elements are the elements in groups 3 – 12 of the periodic table in which the d – orbitals are progressively filled.
1) Magnetic properties : Most of transition elements show paramagnetism due to the presence of unpaired electrons. The magnetic moment (μ)μ=√n(n+2)
2) Formation of coloured ions : Transition elements form coloured compounds due to the presence of unpaired d – electrons, which can take part in d – d transition.
3) Formation of complex compounds: Transition metals form a large number of complex compounds. This is due to the comparatively smaller sizes of the metal ions, their high ionic charges and the availability of d – orbitals for bond formation, e.g., K4[Fe(CN)6], [Co(NH3)6]CI3
4) Catalytic properties : Transition elements and their compounds act as good catalysts. This is attributed to their ability to adopt show multiple oxidation states because of and to form complexes due to the presence of partially filled d – orbitals, e.g., Finely divided Fe is used as a catalyst in Haber’s process.
Question 1.
Account for the following trends in atomic and ionic radii of transition metals. (March – 2013)
i) Ions of the same charge in a given series (3d, 4d or 5d) show progressive decrease in radii with icreasing atomic number.
ii) The atomic radii of elements in 4d series are more than that of corresponding elements in 3d series.
iii) The atomic radii of the corresponding elements in ‘4d’ series and ‘5d’ series are virtually the same.
Answer:
i) This is because each time a new electron enters a ‘d’ orbital the nuclear change increases by unity. The shielding effect of a ‘d’ electron is not that effective. Hence, the net electrostatic attraction between the nuclear charge and the outermost electron increases and the ionic radius decreases.
ii) The effect of addition of new shells in 4d series overtakes the effect of increase in nuclear charge.
Thus, electrostatic attraction between nucleus and valence electron decreases and hence atomic size increases.
iii) This phenomenon is due to the intervention of the 4f – orbitals which must be filled before the 5d series of elements begins. The filling of 4f before 5d – orbitals results in a regular decrease in atomic radii called Lanthanoid Contraction due to imperfect shielding of intervening 4f – orbital electrons which compensate for the expected increase in atomic size on moving down the group, with increasing atomic number. The net result of Lanthanoid Contraction is that the second and the third d series exhibit similar radii.
Question 1.
i) d – Block elements belong to group 3 – 12 in the periodic table, in which the d orbitals are progressively filled. (Say – 2013)
a) What is their common oxidation state?
b) Name two important compounds of transition elements.
c) Transition elements form a large number of complex compounds, why?
ii) What is mischmetal?
Answer:
- a) (n – 1)d1-10 ns1-2
b) Potassium dichromate, Potassium permanganate. KMnO2
c) This is due to the following two factors: Cations of transition metals are very- small in size
- high effective nuclear
- have vacant d – orbitals
- ‘Misch metal’ is an alloy which consists of a lanthanoid metal (- 95%) and iron (- 5%) and traces of S, C, Ca and Al. It is used in Mg-based alloy to produce bullets, shell and lighter flint.
Question 1.
Potassium dichromate is an orange coloured crystal and is an important compound used as an oxidant in many reactions. (March – 2014)
a) How do you prepare K2Cr2O7 from chromite ore?
b) How will you account for the colour of potassium dichromate crystals?
Answer:
a) The chromite ore is fused with sodium carbonate in free access of airto get sodium chromate.
4FeCr2O4+8Na2CO3+7O2⟶8Na2CrO4+2Fe2O3+8CO2
The yellow solution of sodium chromate is filtered and acidified with sulphuric acid to get sodium dichromate.
2Na2CrO4+2H+→Na2Cr2O4+2Na++H2O
The sodium dichromate solution is treated with potassium chloride to get potassium dichromate.
Na2Cr2O7+2KCl→K2Cr2O7+2NaCl
Orange crystals of potassium dichromate crystallise out.
b) This is due to charge transfer spectra i.e., Chromium being a transition element has vacant d – orbitals.
Question 1.
Potassium permanganate and potassium dichromate are two transition metal compounds. (Say – 2014)
a) Write any four characteristics of transition metals.
b) i) Write any two uses of potassium permanganate.
ii) Draw the structure dichromate ion.
Answer:
a)
- Variable oxidation states.
- Formation of coloured ions in aqueous solution.
- Formation of complex compounds.
- Formation of interstitial compounds,
b) i)
- Lab reagent
- For bleaching of wool, cotton, silk and other textile fibres.
ii) The dichromate ion consists of 2 tetrahedra sharing one comer with Cr – O – Crbond angle of 126°.
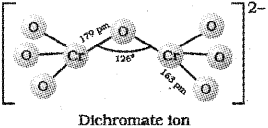
Question 1.
Fourteen elements following Lanthanum are called Lanthanoids: (March – 2015)
a) What is Lanthanoid contraction? Give reason for it.
b) KMnO4 is a purple coloured crystal and it acts as an oxidant. How will you prepare KMnO4 from MnO2?
Answer:
a) The overall decrease in atomic and ionic radii from lanthanum to lutetium is called lanthanoid contraction.
Lanthanoid contraction is caused by the imperfect shielding of one 4f electron by another in the same set of orbitals. The shielding of one 4f electron by another is less than that of one d electron by another. Hence, as the nuclear charge increases along the lanthanoid series, there is fairly regular derease in the size of the entire 4fn orbitals.
b) MnO2 is fused with an alkali metal hydroxide and an oxidising agent like KNO3 to get dark green potassium manganate, K2MnO4.
2MnO2 + 4KOH + O2 → K2MnO4 + 2H2O
Potassium manganate disproportionates in a neutral or acidic solution to give potassium permanganate.
3MnO2-4 + 4H+ → 2MnO4- + MnO2 + 2H2O
Question 1.
a) Which of the following oxidation state is common for lanthanids? (Say – 2015)
i) +2
ii) +3
iii) +4
iv) +5
b) Drawthe structures of chromate and dichromate ions.
c) Zirconium (Zr) belongs to ‘4d’ and Hafnium (Hf) belongs to ‘5d’ transition series. It is difficult to separate them. Explain.
Answer:
a) ii) +3
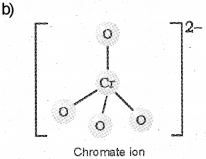
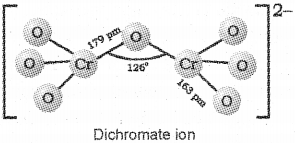
c) It is a consequence of lanthanoid contraction, a cumulative effect of the contraction of atomic radii of the lanthanoid series caused by the imperfect shielding of one electron by another in the 4f sub-shell.
Question 1.
a) Which of the following oxidation state is not shown by Maganese? (March – 2016)
a) +1
b) +2
c) +4
d ) +7
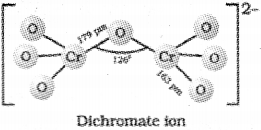
b) Represent the structure of dichromate ion.
c) Potassium permanganate (KMnO4) is a strong oxidizing agent. Write any two oxidizing reactions of KMnO4.
Answer:
a) +1
c) 1. Permanganate ion oxidises iodide to iodine in acid medium:
10|++2MnO4+16H+→2Mn2++8H2O+5I2
2. Permanganate ion oxidises Fe2+ ion (green) to Fe3+ ion (yellow) in acid medium:
5Fe2++MnO4+8H+→Mn2++4H2O+5Fe3+
Question 1.
Transition elements are d-block elements and inner transition elements are f-block elements. (Say – 2016)
i) Write any two properties of transition elements.
ii) Name a transition metal compound and write one use of it.
iii) What is Lanthanoid Contraction?
iv) Write any two consequences of Lanthanoid Contraction.
Answer:
i) Transition elements are metals, have high melting points and high enthalpy of atomisation, exhibit variable oxidation states, show paramagnetism, form coloured compounds, form complex compounds, show catalytic properties, form interstitial compounds, form alloys etc. (any two properties)
ii) Fe to makes steal
iii) The overall decrease in atomic and ionic radii from lanthanum to lutetium, caused by the poor shielding of one 4f electron by another is called lanthanoid contraction.
iv)
1. The atomic radii of second row of transition elements are almost similar to those of third row of transition elements.
2. The almost identical radii of Zr (160 pm) and Hf (159 pm).
3. All the lanthanoids have quite similar properties and due to this they are difficult to be separated.
4. The basic strength of hydroxides decreases from La(OH)3 to Lu(OH)3 due to decrease in size of M3+ ions and consequent increase in the covalent character of M – OH bond.
(any two consequences required)
Question 1.
a) Transition elements are ‘d’ block elements. (March – 2017)
i) Write any four characteristic properties of transition elements.
ii) Cr2+ and Mn3+ have d4 configuration. But Cr2+ is reducing and Mn3+ is oxidising. Why?
b) Which of the following is not a lanthanoid element?
i) Cerium
ii) Europium
iii) Lutetium
iv) Thorium
Answer:
a) i) Transition elements are metals, have high melting points and high enthalpy of atomisation, exhibit variable oxidation states, show paramagnetism, form coloured compounds, form complex compounds, show catalytic properties, form interstitial compounds, form alloys etc. (any four properties)
ii) For Cr2+ to Cr3+ configuration changes from d4 to d3 For Mn3+ to Mn2+ d5 configuration results in extra stability due to half-filled configuration,
b) iv) Thorium
We hope the Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 8 The d and f Block Elements help you. If you have any query regarding Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 8 The d and f Block Elements, drop a comment below and we will get back to you at the earliest.
