Plus Two Chemistry Chapter Wise Previous Questions Chapter 7 The p Block Elements is part of Kerala Plus Two Chemistry Chapter Wise Previous Year Questions and Answers. Here we have given Plus Two Chemistry Chapter Wise Questions and Answers Chapter 7 The p Block Elements.
Kerala Plus Two Chemistry Chapter Wise Previous Questions Chapter 7 The p Block Elements
Question 1.
Elements in the groups 13 to 18 in the Periodic table constitute the ‘p’ block elements. (March – 2010)
i) Name the most important oxo acid of Nitrogen.
ii) How will you prepare the above oxo acid on large scale?
iii) In general, noble gases are least reactive. Why?
Answer:
i) Nitric acid (HNO3)
ii) HNO3 can be prepared on a large scale by Gstwald process. It involves three steps.
a) NH3 is oxidised catalytically by atmospheric oxygen.
b) Nitric oxide thus formed combines with oxy gen giving NO2.
\(2 \mathrm{NO}_{(g)}+\mathrm{O}_{2(g)} \rightleftharpoons 2 \mathrm{NO}_{2(g)}\)C) Nitrogen dioxide so formed dissolves in water to give HNO3.
iii)
- The noble gases except helium (1s2) have completely filled ns2np6 electronic configuration in their valence shell.
- They are octet completed and stable so they are inert.
Question 2.
After a discussion about the structures of hydrides of the group – 15 elements, Neethu wrote the order of bond angles as NH3 < PH3 < AsH3 (Say – 2010)
a) is this the correct order?
b) Justify your answer.
c) Give the hybridization and shape of these hydrides.
d) Also arrange the above hydrides in the increasing order of their thermal stability. Justify your answer.
Answer:
a) No.
b) From top to bottom in the group the bond angle of group 15 hydrides decreases. As the electronegativity of the central atom decreases on moving down the group, the bond pair-bond pair repulsion decreases. Hence the bond angle decreases ¡n the order NH3 > PH3 > AsH3.
C) In all hydrides the central atom is sp3 hybridised. The molecules assume trigonal pyramidal geometry with a lone pair on the central atom.
d) Thermal stability of the group 15 hydrides increases BiH3 < AsH3 < PH3 < NH3 This is due to the fact that moving up the group the E – H bond dissociation enthalpy (‘E’ is a group 15 element) increases due to decrease in size of the central atom and the molecules will decompose only at higher temperatures.
Question 3.
Discovery of Haber’s process for the manufacture of Am monia is considered to be one of the principal discoveries of the twentieth century. (March – 2011)
a) Which is the promoter used ¡ri the earlier process when Iron was used as a catalyst?
b) What is the temperature condition for the maximum yield of Ammonia? Justify.
c) Explain how can you convert NH3 to HNO3, on a large scale commercially.
Answer:
a) Molybdenum (Mo)
b) By Le – Chaltiers principle, the rate of an exothermic reaction increases with decrease in temperature of 500°C to get good yield of product.
C) NH3 is converted to HNO3 commercially by Ostwald’s process. It involves three steps.
i) NH3 is oxidised catalytically by atmospheric oxygen.

ii) Nitric oxide thus formed combines with oxy gen giving NO2.
\(2 \mathrm{NO}_{(g)}+\mathrm{O}_{2(g)} \rightleftharpoons 2 \mathrm{NO}_{2(g)}\)
iii) Nitrogen dioxide so formed dissolves in water to give HNO3.
\(3 \mathrm{NO}_{2(g)}+\mathrm{H}_{2} \mathrm{O}_{(1)} \rightarrow 2 \mathrm{HNO}_{3(\mathrm{aq})}+\mathrm{NO}_{(g)}\)
Question 4.
The phosphorus of group 15 and sulphur of group 16 are two industrially important ‘p’ block elements. Their compounds are also industrially important. (Say – 2011)
a) \(4 \mathrm{H}_{3} \mathrm{PO}_{3} \stackrel{\text { heat }}{\longrightarrow} 3 \mathrm{H}_{3} \mathrm{PO}_{4}+\mathrm{PH}_{3}\) show that this is a disproportionation reaction.
b) PCl3 fumes in moisture. Give reason.
c) Sulphuric acid can be manufactured from sulphur using V2O5 as a catalyst.
i) Give the name of the method.
ii) Outline the principle.
Answer:
a) Disproportionation reactions are a special type of redox reactions in which an element in one oxidation state is simultaneously oxidised and reduced. In phosphorous acid (H3PO3) phosphorus is in the intermediate oxidation state of +3. It is increased to +4 (oxidation) in phosphoric acid (H3PO4) and decreased to – 3 (reduction) in phosphine (PH3).
b) PCI3 undergoes hydrolysis in presence of moisture giving fumes of HCI. PCI3 + 3H2O → H3PO3 + 3HCI
c) i) Contact Process
Question 5.
a) Important allotropic forms of phosphorus are white phosphorus, red phosphorus and black phosphorus. Among these which allotropic form is more reactive? Why? (March – 2012)
b) In the manufacture of sulphuric acid (H2SO4) the final product obtained is oleum.
i) What is Oleum?
ii) Write chemical equation forthe conversion of oleum to sulphuric acid.
c) Name the halogen which forms only one oxoacid and also write the formula of the oxo acid of that halogen.
d) Which element among inert gases form a maximum number of compounds? Write the formula of one of the compounds formed by the element.
Answer:
a) White Qhosfhorus It consists of discrete tetrahedral P4 molecules.
b) i) Pyrosuiphuncacid
ii) H2S2O7 + H2O → 2H2SO4
c) Flounne or HOF or hypoflourous acid
d) Compounds – Xe F2, XeF4 Xe O3, Xe OF2 etc.,
Question 6.
i) What are the products obtained when copper reacts with concentrated nitric acid? (Say – 2012)
ii) Name two important xenon fluorides.
iii) Interhalogen compounds are compounds formed by combination of different halogen atoms. Which are more reactive, halogens or interhalogen compounds? Give reason.
Answer:
i) Copper reacts with concentrated nitric acid to give copper nitrate and nitrogen dioxide.
Cu + 4HNO3(conc) + Cu(NO3)2 + 2NO2 + 2H2O
ii) Two important xenon fluorides are XeF2 and XeF4.
iii) Interhalogen compounds are more reactive than halogens (except fluorine). Because, the bond between different halogen atoms (X – X’) in interhalogen compounds is weakerthan the bond between similar halogen atoms. Due to the difference in size and electronegativity. The F – F bond is the weakest due to interelectronic repulsion.
Question 7.
a) Nitrogen forms a number of oxides in the different oxidation stats. Write the names and structural formulae of any four oxides of nitrogen. (March – 2013)
b) Boiling point of H2O (373 K) is very much higher than that of H2S (213k). Give reason.
c) Suggest a method for the quantitative estimation of ozone (O3).
Answer:
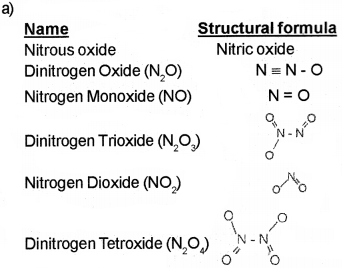
b) Molecules of water are highly associated through hydrogen bonding resulting in its high boiling point. Hydrogen bonding is not possible ¡n H2S.
c) When O3 reacts with an excess of Kl solution buffered with a borate buffer (pH = 9.2) 12 is liberated, which can be titrated against a standard solution of sodium thio sulphate. 2Kl+ H2O + O3 → O2 + KOH + l2
Question 8.
a) Name the products obtained when the copper reacts with concentrated nitric acid. (Say – 2013)
b) Write down the chemical reaction between concentrated nitric acid and aluminium.
c) What is the basicity of H3PO3?
d) How do you account for the basicity of H3PO3?
e) Write down the three steps involved in the manufacture of sulphuric acid by the Contact Process.
f) Write any two important uses of noble gas elements.
Answer:
a) Copper nitrate, Nitrogen dioxide and Water. [Cu + 4HNO3(conc) → Cu(NO3) + 2NO2 + 2H2O]
b) Aluminium does not dissolve in concentrated nitric acid because it is rendered passive due to the formation of a thin protective layer of metal oxide on the surface of the metal which cuts off the further action.
c) T’ do.
d) The basicity of oxo acids of phosphors is deter mined by the number of P – OH bonds, because only those H atoms which are attached with oxygen ¡n P – OH form are ionisable and cause the basicity. H3PO3 has two P – OH bonds.
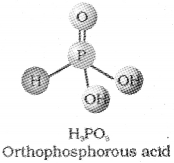
e) i) Preparation of suiphurdioxide by burning sulphur. S(s) + O2(g) → SO2(g)
ii) Oxidation of sulphur dioxide to suphurtrioxide catalytically with atmospheric oxygen.

iii) Preparation of oleum by absorbing sulphur trioxide in sulphuric acid. It is diluted with enough water to get sulphuric acid of desired concentration.
\(\begin{array}{l}
\mathrm{SO}_{3}(\mathrm{~g})+\mathrm{H}_{2} \mathrm{SO}_{4}(\mathrm{I}) \rightarrow \mathrm{H}_{2} \mathrm{~S}_{2} \mathrm{O}_{7}(\mathrm{I}) \\
\mathrm{H}_{2} \mathrm{~S}_{2} \mathrm{O}_{7}(\mathrm{I})+\mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \rightarrow \mathrm{H}_{2} \mathrm{SO}_{4}(\mathrm{aq})
\end{array}\)
Orthophosphorous acid Neon is used in discharge tubes and fluorescent bulbs for advertisement display purposes. Argon is used to provide an inert atmosphere in high-temperature metallurgical processes.
Question 9.
Compounds of nitrogen, phosphorus and sulphur such as ammonia, phosphoric acid and sulphuric acid are used in the fertilizer industry. (March – 2014)
a) Describe Haber process for the manufacture of ammonia.
b) Write the chemical equation forthe preparation of phosphoric acid (H3PO4) from ortho phosphorous acid (H3PO3).
c) Describe contact process for the manufacture of sulphuric acid.
Answer:
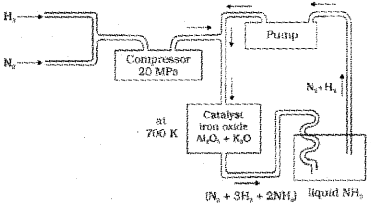
a) On a large scale, ammonia is manufactured by Haber’s process. In this process nitrogen gas reacts with hydrogen gas to form ammonia gas as per the reaction:
\(\mathrm{N}_{2(g)}+3 \mathrm{H}_{2(g)}=2 \mathrm{NH}_{3(g)} ; \Delta_{f} \mathrm{H}^{\circ}=-46.1 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
According to Le Chatelier’s principle, high pressure favours the formation of ammonia. The optimum conditions for the production of ammonia are a pressure of 200 x 105 Pa (about 200 atm), a temperature of 700 K and the use of catalysts such as iron oxide with small amounts of K2O and Al2O3 to increase the rate of attaintment of equilibrium. The flow chart for the production of ammonia is shown below:
b) Orthophosphorous acid on heating disproportionates to give orthophosphoric acid and phosphine.
4H3PO3 → 3H3PO4 + PH3
c) i) Bunning of sulphur or sulphide ores in air to generate SO2. S(s) + O2(g) → SO2(g))
ii) Conversion of SO2 to SO3 by the reaction with oxygen in the presence of V2O5 catalyst.
\(\begin{array}{l}
2 \mathrm{SO}_{2(\mathrm{~g}}+\mathrm{O}_{2(\mathrm{~g})} \stackrel{\mathrm{V}_{2} \mathrm{O}_{3}}{\longrightarrow} 2 \mathrm{SO}_{3(\mathrm{~g})} \Delta_{\mathrm{r}} \mathrm{H}^{\circ} \\
=-196.6 \mathrm{~kJ} \mathrm{~mol}^{-1}
\end{array}\)
A pressure of 2 bar and a temperature of 720 K are applied.
iii) Absorption of SO3 gas in H2SO4 to give oleum (H2S2O7) SO3 + H2SO4 → H2S2O7 Dilution of oleum with water gives H2S04 of the desired concentration. H2S2O8 + H2O → 2H2SO4
Question 10.
Ammonia and Nitric acid are two industrially mportant compounds. (Say – 2014)
a) Write any two uses of ammonia.
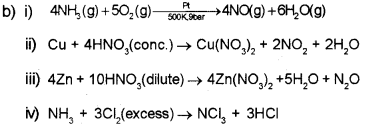
b) Complete the following equations. (Balancing is not required)
i) NH3 + O2 > \(\frac{\mathrm{Pt}}{500 \mathrm{~K}, 9 \mathrm{ber}}]\)
ii) Cu + Con. HNO3 →
iii) Zn + dil. HNO3 →
iv) NH3 + excess Cl2 →
OR
a) Phosphorus forms a number of oxoacids. Write the name or formulae of any two dibasic oxoacids of phosphorus.
b) Account for the following:
i) PCl3 fumes in moist air.
ii) Nitrogen does not form a pentahalide.
iii) Boiling point of PH3 is less than that of NH3
iv) NO2 undergone dimerisation.
Answer:
a)
- To produce various nitro geneous fertilisers.
- In the manufacture of some inorganic nitrogen compounds like nitric acid.
OR
a) i) Orthophosphorous acid (H3 PO3)
ii) Pyrophosphorous acid (H4P2O5)
b) i) PCI3 hydrolyses in the presence of moisture giving fumes of HCI. PCl3 + 3H2O → H3PO3 + 3HCI
ii) It does not have ‘d’ orbitais to expand its covalence beyond four. That is why it does not form pentahalide.
iii) Unlike NH3, PH3 molecules are not associated through hydrogen bonding in liquid state. That is why the boiling point of PH3 s lower than that of NH3.
iv) NO2 contains odd number of valence electrons. It behaves as a typical odd electron molecule. On dimerisation, it is converted to stable N2O4 molecule with even number of electrons.
Question 11.
Some elements in p – block shows allotropy. (March – 2015)
a) What are the allotropic forms of sulphur?
b) i) How will you manufacture Sulphuric Acid by contact process?
ii) What are interhalogen compounds?
Answer:
a) Rhombic sulphur (α – sulphur) and Monoclinic sulphur (β – sulphur)
b) i) The manufacture of sulphuric acid by contact process involves three steps:
1) Burining of sulphur or sulphide ores in air to generate SO2. S(s) + O2(g) → SO2(g)
2) Conversion of SO2 to SO3 by the reaction with ozygen in the presence of V2O5 catalyst.
\(2 \mathrm{SO}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \frac{\mathrm{V}_{2} \mathrm{O}_{5}}{2 \mathrm{~S} \mathrm{O}_{3}(\mathrm{~g}) \mathrm{\Delta}_{\mathrm{r}} \mathrm{H}^{\circ}}=-196.6 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
This reaction is exothermic, reversible and the forward reaction leads to a decrease in volume. Therefore, low temperature and high pressure are the favaourable conditions for maximum yield. In practice, a pressure of 2 bar and a temperature of 720 K are applied.
3) Absorption of SO3 gas in H2SO4 to give oleum (H2S2O7) SO3 + H2SO4 → H2S2O7 Dilution of oleum with water gives H2S04 of the desired concentration. H2S2O8 + H2O → 2H2SO4
The flow diagram for the manufacture of sulphuric acid by Contact Process is
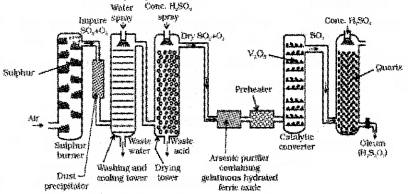
ii) Compounds formed by the reaction between two different halogens are called interhalogen compounds. They can be assigned general compositions as XX’, XX3’, XX5 and XX7’ where X is halogen of larger size and X’ of smaller size and X is more electropositive than X’.
Question 12.
a) Name two oxoacids of Sulphur.
b) i) How will you manufacture ammonia by Haber process?
ii) Write any two uses of inert gases.
Answer:
a) Sulphurous acid (H2S2O5), Sulphuric acid (H2SO4), Peroxodisuiphuric acid (H2S2O5), Pyrosulphunc acid or Oleum (H2S2O7) – Any two.
b) i) Ammonia is manufactured by Haber’s process. In this process nitrogen gas reacts with hydrogen in the ratio 1:3 to form ammonia: \(\mathrm{N}_{2}(\mathrm{~g})+3 \mathrm{H}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NH}_{3}(\mathrm{~g}) ; \Delta_{f} \mathrm{H}^{\odot}=-46.1 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
High pressure favours the formation of ammonia 200 atm, a temperature of 700 K and the use of catalysts such as iron oxide.
ii) Helium is used for filling balloons for meteorological observations, Neon is used in discharge tubes and fluorescent bulbs. Argon is used to provide an inert atmosphere in high-temperature metallurgical processes and for filling electric bulbs, Xenon and Krypton are used in light bulbs designed for special purposes. (Any two)
Question 13.
a) What are interhalogen compounds? Write any two examples. (Say – 2015)
b) Write a method of preparation of phosphine from white phosphorus.
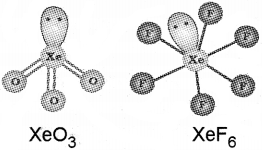
c) Write the name or formula of oxo acid of chlorine, in which chlorine possess oxidation number +7. Draw the structure of XeO3 and XeF2.
Answer:
a)These are compounds formed by the reaction of two different halogens. They can be assigned general compositions as XX’, XX, XX and XX where X is halogen of larger size and X’ of smaller size and X is more electropositive than X’. e.g. dF, dF3, BrF5, IF7 (any two)
b) Phosphifle is prepared by heating white phosphorus with concentrated NaOH solution in an inert atmosphere of CO2.
P4 + 3NaOH + 3H2O → 4 PH3 + 3NaH2PO2
c) Perchloric acid or Chionc (VII) acid (HOCIO3)
d)
Question 14.
a) Account for the following: (March – 2O16)
i) NH3 acts as a Lewis base.
ii) PCI3 fumes ¡n moist air.
iii) Fluorine shows only – 1 oxidation state.
b) i) SuggestanytwofluoridesofXenon.
ii) Write a method to prepare any one of the above mentioned Xenon fluorides.
OR
a) Account for the following:
i) H2O is a liquid while H2S is a gas.
ii) Noble gases have very low boiling points.
iii) NO2 dimerises to N2O4.
b) i) What are interhalogen compounds?
ii) Suggest any two examples of interhalogen compounds.
Answer:
a) i) Nitrogen atom in NH3 has one lone pair of electrons which is available for donation. There fore, it acts as a Lewis base.
ii) PCI3 hydrolyses in the presence of moisture giving fumes of HCI. PCI3 + 3H2O → H3PO3 + 3HCI
iii) Fluorine is the most electronegative element and cannot exhibit any positive oxidation state. Fluorine atom has no d orbitals in its valence shell and therefore cannot expand its octet.
b) i) Xenon ditluoride, XeF2
Xenon tetrafluoride, XeF4
Xenon hexafluonde, XeF6 (any two)
ii) XeF2 is prepared by treating Xe with excess fluorine at 673 Kandl bar.
\(\mathrm{Xe}(\mathrm{g})+\mathrm{F}_{2}(\mathrm{~g}) \longrightarrow 673 \mathrm{~K}, 1 \text { bar } \quad>\mathrm{XeF}_{2}(\mathrm{~s})\)
Or, XeF4 is prepared by treating Xe with excess fluorine in 1: 5 ratio at 873 K and 7 bar.
\(\mathrm{Xe}(\mathrm{g})+2 \mathrm{~F}_{2}(\mathrm{~g}) \longrightarrow \mathrm{B} 73 \mathrm{~K}, 7 \mathrm{bar} \quad \rightarrow \mathrm{XeF}_{4}(\mathrm{~s})\)
Or, XeF6 is prepared by treating Xe with excess fluorine in 1 :20 ratio at 573 K and 60 – 70 bar.
\(\mathrm{Xe}(\mathrm{g})+3 \mathrm{~F}_{2}(\mathrm{~g}) \longrightarrow 573 \mathrm{~K}, 60-70 \mathrm{bar} \quad \longrightarrow \mathrm{XeF}_{6}(\mathrm{~s})\)
OR
a) i) Due to small size and high electronegativity of oxygen it is capable of forming hydrogen bond. Thus, water molecules can associate through intermolecular hydrogen bonds and hence ¡t exists as a liquid.
Due to big large and low electronegativity of sulphur t is not capable of forming hydrogen bond. So hydrogen sulphide molecules cannot associate through intermolecular bonds and hence it exists as a gas.
ii) Noble gases being monoatomic have no interatomic forces except weak dispersion forces and therefore, they are liquefied at very low temperatures. Hence, they have low boiling points.
iii) NO2 contains odd numberof valence elecrons. It behaves as a typical odd electron molecule. On dimensation, it is converted to stable N2O4 molecule with even number of electrons.
b) i) These are compounds formed by the reaction between two different halogens.
ii) dF, BrF, IF, BrCI, ICI, dF3, BrF3, IF3, ICI3, IF5,
BrF5, dF5, IF7 (any two)
Question 15.
Nitrogen shows different oxidation states in different oxides. (Say – 2016)
a) In which of the fof lowing oxides, nitrogen is in + 4 oxidation state?
a) NO
ii) N2O
iii) N2O3
iv) NO2
b) Prepare a short write upon Nftric acid highlighting its structure, manufacture and any two properties.
OR
Phosphorous forms oxoacids
a) In which of the following phosphorous is in + 1 oxidation state?
i) H3PO2
ii) H3PO3
iii) H4P2O7
iv) H3PO4
b) Prepare a short write up on Ammonia highlighting its structure, manufacture and properties.
Answer:
a) iv) NO2
b) Nitric acid is the most important oxoacid of nitrogen. HNO3 exists as planar molecule.
Manufacture of nitric acid: On a large scale, nitric acid is prepared mainly by Ostwald’s process. This method is based upon catalytic oxidation of NH3 by atmospheric oxygen.
4NH3(g) + \(5 \mathrm{O}_{2}(\mathrm{~g}) \frac{\text { PUR } \text { guage catalyst }}{500 \mathrm{~K}, \text { bar }}\) 4NO(g) + 6H2O(g)
Nitric oxide thus formed combines with oxygen giving NO2.
2NO(g) + O2(g) \(\rightleftharpoons\) 2NO2(g)
Nitrogen dioxide so formed, dissolves in water to give HNO2.
3NO2(g) + H2O(l) → 2HNO3(aq) + NO(g)
Properties of nitric acid: It is a colourless liquid. In aqueous solution nitric acid behaves as a strong acid giving hydronium and nitrate ions.
HNO2(g) + H2O(1) → H3O+(aq) + NO3(aq)
Concentrated nitric acid is a strong oxidising agent and attacks most metals except noble metals such as gold and platinum.
OR
a) i) H3PO2
b) Structure of ammonia: The ammonia molecule is trigonal pyramidal with the nitrogen atom at the apex. It has three bond pairs and one lone pair of electrons.
Manufacture of ammonia: On a large scale ammonia is manufactured by Haber’s process.
\(\mathrm{N}_{2}(\mathrm{~g})+3 \mathrm{H}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NH}_{3}(\mathrm{~g}) ;=-46.1 \mathrm{~kJ} \mathrm{~mol}^{-1}\)High pressure would favour the formation of ammonia. (about 200 atm), a temperature of —700 K and the use of catalyst such as iron oxide with small amounts of K2O and Al2O3.
Ammonia gas is highly soluble in water. Its aqueous solution is weakly basic due to the formation of OH ions.
\(\mathrm{NH}_{3(\mathrm{~g})}+\mathrm{H}_{2} \mathrm{O}_{(0)} \rightleftharpoons \mathrm{NH}_{4^{+}(\mathrm{aq})}+\mathrm{OH}_{-(\mathrm{aq})}\)It forms salts with acids. It precipitates the hydroxides of many metals from their salt solutions. The presence of a lone pair of electrons on the nitrogen atom of the ammonia molecule makes it a Lewis base.
Question 16.
Nitrogen forms a number of oxides and oxoacids. (March – 2017)
a) Which of the following is a neutral oxide of Nitrogen.
i) N2O
ii) N2O5
iii) NO2
iv) N2O4
b) Prepare a short write – up on Nitric acid high lighting its laboratory preparation, chemical properties and uses.
OR
Phosphorous forms a number of compounds.
a) The gas liberated when calcium phosphide is treated with die. HCl is
i) Cl
ii) H2
iii) PH3
iv) All the above
b) Prepare a short write up on PCl3 and PCI5 highlighting the preparation and chemical properties of PCl3 and structure of PCl5.
Answer:
a) i) N2O
b) Laboratory preparation: In the laboratory, nitric acid is prepared by heating KNO3 or NaNO3 and concentrated H2SO4 in a glass retort.
NaNO3 + H2SO4 → NaHSO4 + HNO3
Uses: in the manufacture of ammonium nitrate for fertilisers and other nitrates for use in explosives and pyrotechnics; for the preparation of nitroglycerin, trinitrotoluene and other organic nitro compounds; in the pickling of stainless steel etching of metals and oxidiser in rocket fuels.
OR
a) iii) PH3
b) Preparation of PCI3: By passing dry chlorine over heated shite phosphorus.
P4 + 6Cl2 → 4PCl3
Or, by the action of thionyl chloride with white phosphorus.
P4 + 8SOCl2 → 4PCI3 + 4SO2 + 2S2Cl2
Properties of PCI3: It is a colourless oily liquid and hydrolyses in the presence of moisture. Hence, it fumes in moist air.
PCI3 + 3H2O → H3PO3 + 3HCI
Structure of PCI5: In gaseous and liquid phases, PCI5 has a trigonal bipyramidal structure. The three equational P – CI bonds are equivalent, while the two axial bonds are longer than equatorial bonds. This is due to the fact that the axial bond pairs suffer more repulsion as compared to equatorial bond pairs.
Question 17.
a) Identify the most acidic compound from the following (Say – 2017)
i) H2O
ii) H2S
iii) H2Se
iv) H2Te
b) ![]()
i) Explain the step P and Q.
ii) Give a reaction which indicates dehydration property of conc. H2SO4.
iii) Write any two uses of sulphuric acid.
OR
a) Identify the least basic compound among the following:
i) NH3
ii) PH3
iii) AsH3
iv) SbH3
b) i) Halogens have maximum negative electron gain enthalpy in the respective periods. Give reason.
ii) Draw the structure of Perch bric acid (HClO4)
iii) Write the formulae of any two interhalogen compounds.
Answer:
a) iv or H2Te
b) i)
ii) Charring action of cane sugar to carbon
C12H22O11 + Con H2SO4 → 12 C+ 11 H2O
iii) Dehydrating agent, laboratory reagent
OR
a) iv) SbH3
b) i) by getting one electron octet ¡s completed. So electronegative ¡s very high
We hope the Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 7 The p Block Elements help you. If you have any query regarding Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 7 The p Block Elements, drop a comment below and we will get back to you at the earliest.
