Plus Two Chemistry Chapter Wise Previous Questions Chapter 4 Chemical Kinetics is part of Kerala Plus Two Chemistry Chapter Wise Previous Year Questions and Answers. Here we have given Plus Two Chemistry Chapter Wise Questions and Answers Chapter 4 Chemical Kinetics.
Kerala Plus Two Chemistry Chapter Wise Previous Questions Chapter 4 Chemical Kinetics
Question 1.
The order of a chemical reaction can be zero and even a fraction but molecularity cannot be zero or a non-integer. (March – 2010)
i) What do you mean by the order of a reaction?
ii) What is the molecularity of a reaction?
iii) The conversion of molecules ‘A’ to ‘B’ follows second order kinetics. If the concentration of A is increased to three times, how will it affect the rate of formation of ‘B’?
Answer:
i) It is sum of powers of the concentration – terms of the reactants in the rate law expression.
ii) The number of reacting species in an elementary reaction.
iii) Increases by 9 times.
Rate = k[R]2
Rate’= k[3R]2 = 9 [R]2; i.e., Rate = 9 Rate
Question 2.
In a class room discussion about order and molecularity of a chemical reaction, Ramu argued that “there are reactions which appear to be of higher order but actually follow first order kinetics”. (Say – 2010)
a) How far is his statement true? Give your opinion in this regard. Justify your answer using suitable example.
b) List out any three important differences between order and molecularity.
Answer:
a) The reaction appears to be of higher order but actually follows a lower order kinetics. Such reactions are called pseudo order reactions. For example, hydrolysis of ethyl acetate. This reaction appears to be of second order but actually follows first order kinetics.
Here the concentration of water does not get altered much during the course of the reaction.
Hence, [H2O] can be taken as a constant. The equation thus becomes
Rate = k[CH3CQOH] where k = k [H2O]
Thus, the reaction behaves as a first order reaction.
(b)
| Order | Molecularity |
| i. Experimental | i. Theoretical |
| ii Can be zero | ii Can not be zero |
| iii Can be a fraction | iii Cannot be a fraction |
Question 3.
The hydrolysis of an ester in acid medium is a first-order reaction. (March – 2011)
a) What do you mean by a first-order reaction?
b) What is the relation between Rate Constant and Half-Life Period of a reaction?
c) Half-Life Period of a first-order reaction is 20 seconds. How much time will it take to complete 90% of the reaction?
Answer:
a) When the sum of the powers of the concentra¬tion terms in the rate expression is one, that reaction is called a first-order reaction.
Rate = k[A]1
b) In the case of the first-order reaction,
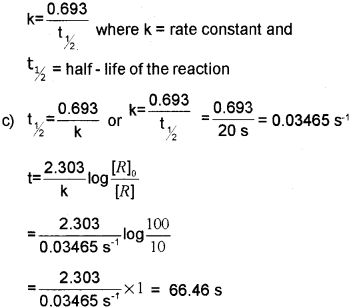
Question 4.
The value of rate constant K of a reaction depends on temperature. From the values of K at two different temperatures, the Arrhenius parameters Ea, and A can be calculated. (Say – 2011)
a) The rate constant of a reaction at 600K and 900K are 0.02 s-1 and 0.06 s-1 respectively. Find the values of Ea and A.
b) Write the unit of rate constant ‘K’ of a reaction if the concentration is in mol L-1 and time in s. (order of the reaction is two)
Answer:
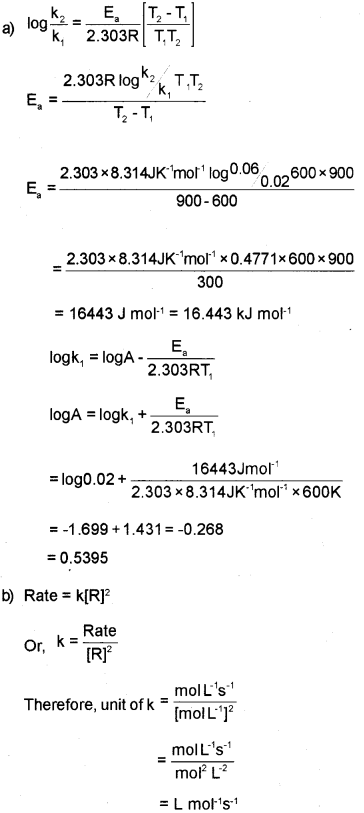
Question 5.
Rate of a reaction is the change in concentration of any one of the reactants or any one of the products in unit time. (March – 2012)
i) Express the rate of the following reaction in terms of reactants and products:
2H I → H2 + l2
ii) If rate expression for the above reaction is, rate = k[H I]2, What is the order of the reaction?
iii) Define order of a reaction.
iv) Whether the molecularity and the order of the above reaction are the same ? Give reason.
Answer:
i) In terms of reactants
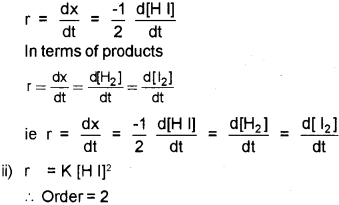
iii) Order is the sum of the powers of the concentration terms in the rate law.
rate = K [A]x [B]y
∴ Order = x + y
iv) Yes.
2H I = H2 + l2
rate = K [H I]2 ∴ Order = 2
Molecularity is the number of reacting species taking part in an elementary reaction which must colloide simalteneously in order to bring about a chemical reaction.
∴ Here molecularity = 2
Question 6.
For a first order reaction half life period (t1/2) is independent of initial concentration of its reacting species. (Say – 2012)
i) What is meant by half life period of a reaction?
ii) By deriving the equation for t1/2 of first order reaction, prove that it is independent of initial concentration of its reacting species.
[Hint: Fora first ortler reaction, k=2.303tlog[R]0[R]]
Answer:
i) The half-life period of a reaction is the time in which the concentration of a reactant is reduced to one half of its initial concentration,
ii) For a first order reaction,
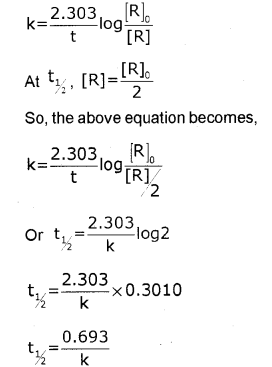
Thus, for a first order reaction, half-life period is constant, i.e., it is independent of initial concentration of the reacting species.
Question 7.
a) Zero order reaction means that the rate of a reaction is independent of the concentration of reactants. (March – 2013)
i) Write an example for a zero order reaction.
ii) Write the integral rate expression for the zero order reaction, R→ P.
b) The temperature dependence of the rate of a chemical reation can be accurately explained by Arrhenius equation. With the help of Arrhenius equation calculate the rate constant for the first order reaction C2H5l(g) → C2H4(g) + Hl(g) at 700K. Energy of activation (Ea) for the reaction is 209 kJ moh1 and rate constant at 600 K is 1.60 x 10-5 S-1 [Universal gas constant R = 8.314 JK-1 mol-1]
Answer:
a) i) Zero-order reaction
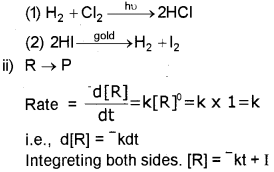
where ‘I is the constant of integration.
At t = 0 1 = [R]0
[R]0 → InitiaI concentration of the reactant
[R] → concentration at time
∴ Equation (1) becomes. [R] = –kt + [R]0
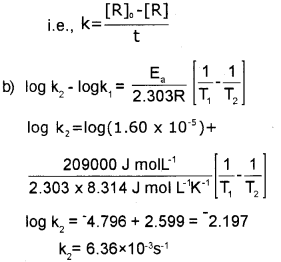
Question 8.
The conversion of molecule A to B follows second order kinetics. (Say – 2013)
a) If the concentration of A is increased to 4 times, how will it affect the formation of B?
b) Indicate the order and molecularityofthe reaction given below.
C12H22O11+H2OH⟶C6H12O6+C6H12O6
Answer:
a) Rate r,= k[A]2
If the concentration of A is increased by four times, the new rate, r2 = k[4A]2
or r2 = 16 k [A]2
or r2 = 16 r1
i.e., rate is increased by 16 times.
b) This is a pseudo first order reaction.
Order = 1, Molecularity = 2
Question 9.
a) Consider a general reaction aA + bB → cC + dD. The rate expression for the reaction is Rate = K[A]*[B]y (March – 2014)
i) Establish the significance of ‘(a+b)’ and ‘(x+y)’ in terms of order and molecularity.
ii) Write any two differences between order and molecularity.
b) “Reactions with zero order is possible, but zero molecularity is not”. Justify the statement.
Answer:
a) i) (a + b) – Molecularity of the reaction
(X + y) – Order of the reaction
ii)
| Order | Molecularity |
| 1. Sum of the powers of the concentration of the reactants in the rate law expression | 1. No. of reacting species taking part in an elementary reaction, which must collide simultaneously to bring about a chemical reaction |
| 2. Experimental quantity | 2. Theoretical concept |
b) The order of a reaction can be zero which means that the rate of the reaction is independent of the concentration of the reactants. But, molecularity of a reaction cannot be zero which means that there is no reacting species and hence no reaction is possible.
Question 10.
a) Unit of rate constant (K) of a reaction depends on the order of the reactions. (Say – 2014)
Values of ‘K’ of two reactions are given below. Find the order of each reaction.
i) K = 3 x 10-2 molL-1 s-1
ii) K = 5 x 10-3 mol-1 Ls-1
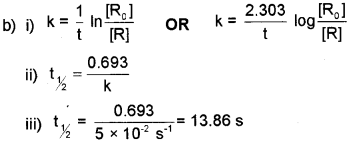
b) i) Write integrated rate equation for a first order reaction.
ii) Write the relation between half life (t1/2) and rate constant (K) of a first order reaction.
iii) Rate constant (K) of a reaction is 5 x 10.2 s-1.
Find the half life (t1/2) of the reaction.
Answer:
a) i) Zero-order reaction by analysing the unit.
ii) Second-order reaction
Question 11.
The terms order and molecularity are common in chemical kinetics. (March – 2015)
a) What do you mean by order and molecularity?
b) i) Write two factors influencing rate of a reaction.
ii) WnteArrhenius equation.
Answer:
a) Order of a chemical reaction is the sum of powers of the concentration of the reactants in the rate law expression.
Molecularity of a reaction is the number of reating species (atoms, ions or molecules) taking part in an elementary reaction, which must collide simultaneously in order to bring about a chemical reaction.
b) i) Temperature, Nature of the reactant, Concentration of the reactant (Pressure in the case of gases). Presence of catalyst, Presence of radiation/light, Surface area etc. (any one)
ii) k=Ae−E0RT OR lnk=−EaRT+lnAQuestion 12.
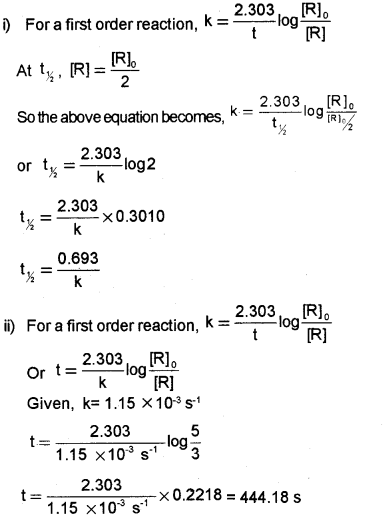
Integrated rate expression for rate constant of first-order reaction is given by K=2.303tlog[R]0[R], for a general reaction R → R (Say – 2015)
i) Derive an expression for half life period of first order reaction.
ii) A first order reaction has a rate constant 1.15 x 10-3s-1. How long will 5g of the reactant take to reduce 3 g?
Answer:
Question 13.
i) The molecularity of the reaction 2NO + O2 → 2NO2 is, (March – 2016)
a) 5
c) 2
c) 3
d) O
ii) a) What do you mean by rate oía reaction?
b) What will be the effect of temperature on rate of a reaction?
iii) A first order reaction is found to have a rate constant, k = 5.5 x 10-14 s-1. Find out the half-life of the reaction.
Answer:
c) 3
ii) a) The rate of a reaction is defined as the change in concentration of any one of the reactants or products ¡n unit time. i.e.,
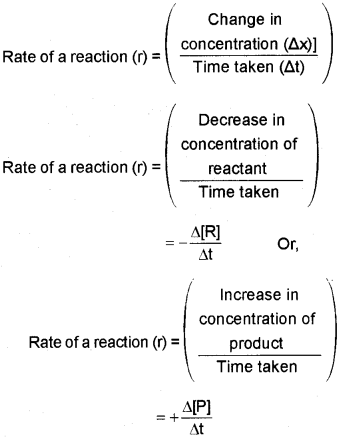
b) The rate of most of the chemical reactions (endothermic reactions) increase with increase in temperature. For a chemical reaçtion with rise ¡n temperature by 100, the rate constant is nearly doubled. The temperature dependence of the rate of a chemical reaction can be accurately explained by Arrhenius equation, k=Ae−E0RT
iii) For a first order reaction, half-life period,
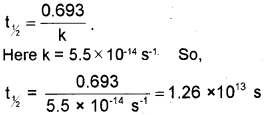
Question 14.
Rate of a reaction is the change in concentration of any one of the reactants or any one of the products in unit time (Say – 2016)
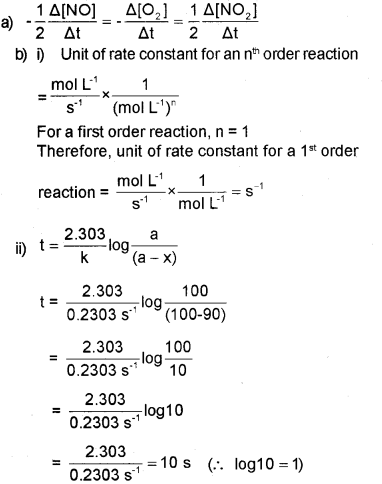
a) Express the rate of the following reaction in terms of reactants and products 2NO(g)+O2(g)→2NO2(g).
b) i) N2O5(g)→2NO2(g)+12O2(g) is a first order reaction. Find the unit of K.
ii) Calculate the time required for the completion of 90% of a first order reaction. (K = 0.2303s-1)
Answer:
Question 15.
a) Plot a graph showing variation in the concentration of reactants against time for a zero-order reaction. (March – 2017)
b) What do you mean by zero-order reaction?
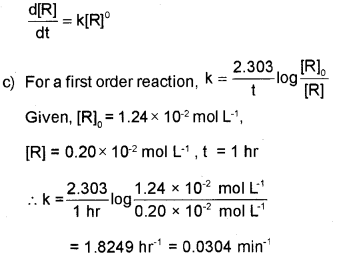
C) The initial concentration of the first-order reaction, N2O5( g)→2NO2( g)+1/2O2(g) was 1 24 x 10-2 mol L-1 at 300 K. The concentration of N2O5 after ‘1’ hour was 0.20 x 10-2 mol L-1. Calculate the rate constant of the reaction at 300 K.
Answer:
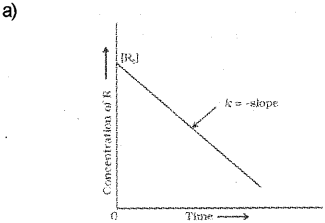
b) It is a reaction for which the rate of the reaction is proportional to zero power of the concentration of reactants, i.e, order is zero.
Or, this is a reaction for which the rate of the reaction is independent of the concentration of the reactants.
Or, this is a reaction for which rate of the reaction,
Question 16.
The effect of temperature on rate of reaction is given by Arrhenius equation. (Say – 2017)
i) Write Arrtenius equation.
ii) Define activation energy (Ea).
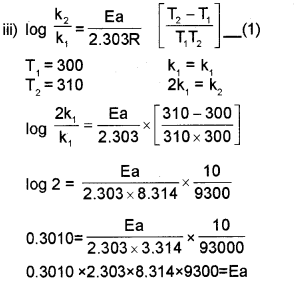
iii) Rate constant K2 of a reaction at 310 K is two times of its rate constant K., at 300 K. Calculate activation energy of the reaction. (1og2 0.3010, log 1=0)
Answer:
I) K=Ae−Ea/RT or logK=logA−Ea2.303RT
ii) Activation energy is the energy required to form an activated complex or It is the energy differ ence between activated complex and the reactant molecules.
We hope the Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 4 Chemical Kinetics help you. If you have any query regarding Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 4 Chemical Kinetics, drop a comment below and we will get back to you at the earliest.
