Plus Two Chemistry Chapter Wise Previous Questions Chapter 3 Electrochemistry is part of Kerala Plus Two Chemistry Chapter Wise Previous Year Questions and Answers. Here we have given Plus Two Chemistry Chapter Wise Questions and Answers Chapter 3 Electrochemistry.
Kerala Plus Two Chemistry Chapter Wise Previous Questions Chapter 3 Electrochemistry
Question 1.
From the position of elements in the electrochemical series, copper (Cu) can displace silver (Ag) from silver nitrate solution. (March – 2010)
a) Represent the cell constructed with silver and copper electrodes.
b) Write down the reaction taking place at the anode.
c) Write down the reaction taking place at the cathode.
d) Write the Nernst equation for the above cell reaction.
Answer:

Question 2.
In a class room, the teacher has explained the quantitative aspects of electrolysis by stating the Faraday’s laws of electrolysis. (Say – 2013)
a) State the Faraday’s laws of electrolysis.
b) Explain the term electrochemical equivalent.
c) Calculate the quantity of electricity required to deposit 0.09 g of Aluminium during the following electrode reaction:
Al3+ + 3e ? Al (Atomic mass of Al = 27)
Answer:
a) First law : The amount of a substance which is deposited or liberated at any electrode during ectrolysis is directly proportional to the quantity of electricity flowing through the electrolyte.
Second law: If the same quantity of electricity is passed through different electrolytes the amount of substances formed is directly proportional to their chemical equivalent weights.
b) It is the quantity of a substance formed when one-ampere current is passed through an electrolyte for one second.
c) Quantity of electricity required to deposit 27 g of
Al = 3F = 3 x 96500 C = 289500 C
? the quantity of electricity required to deposit 0.09
\(g \text { of } A l=\frac{289500 \times .09}{27}=965 C\)
Question 3.
The limiting molar conductivity of an electrolyte is to Aained by adding the limiting molar conductivities of cation and anion of the electrolyte. (March – 2011)
a) Name the above law.
b) What is meant by limiting molar conductivity?
c) Explain how conductivity measurements help to determine the ionization constant of a weak electrolyte like Acetic Acid.
d)Explain the change of conductivity and molar conductivity of a solution with dilution.
Answer:
a) Kohlrauschs law.
b) It is the conductivity of an electrolyte when the concentration of the solution approaches zero (or at infinite dilution).
c) The limiting molar conductivity of acetic acid \(\left(\Lambda_{\mathrm{Gh}_{3} \mathrm{COOH}}\right)\) isdeterrnined by app’ying Kohlrauschts law \(\left(\Lambda_{\mathrm{CH}_{3} \mathrm{COOH}}^{0}=\Lambda_{\mathrm{CH}_{3} \mathrm{COONa}}^{0}+\Lambda_{\mathrm{HCl}}^{0}-\Lambda_{\mathrm{NaCl}}^{0}\right)\). Then, degree of dissociaijon,OE is determined using the re lation, \(a=\frac{\Lambda_{\mathrm{CH}_{3} \mathrm{COOH}}^{\mathrm{C}}}{\Lambda_{\mathrm{CH}_{3} \mathrm{COOH}}^{\mathrm{O}}}\)
From a, the diissociation constant can be deter mined using the relation, \(K_{a}=\frac{c a^{2}}{(1-\alpha)}\)
Substituting for CL we get,
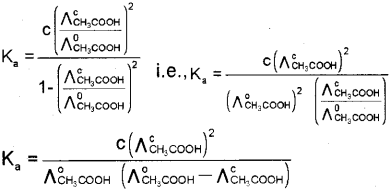
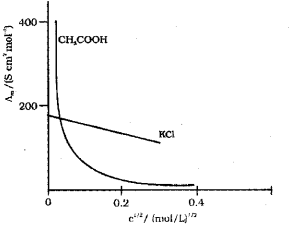
d. Conductivity decreases with dilution because the number of ions per unit volume that carry the current in a solution decreases on dilution. The vanation of molar conductivity with dilution is different for strong and weak electrolytes. For strong electrolytes molar conductivity in creases steadily with increase in dilution due to decrease in interionic attraction. Thus, a straight line is obtained when ?m is plotted against C1/2. For weak electrolytes molar conductivity increases with dilution steadily intially and shows a steep increase, especially at lower concentration due to increase in degree of dissociation.
Thus, a plot of ?m against C1/2 gives a curve,
Question 4.
The standard electrode potentials of some electrodes are given below: (Say – 2011)
E°(zn2+,zn) = – 0.76V
E°(cu2+, Cn) = + 0.34V
E°(Ag+, Ag) = + 080V
E°(H+, H2) = 0V
a) Can CuSO4 solution be kept in silver vessel?
b) Zinc or Copper which can displace hydrogen from dii. H2SO4?
c) What is the reaction taking place at SHE when its connected to Ag/Ag electrode to form a galvanic cell?
d) Find the value of KC (equillibnum constant) in the Daniel cell at 298k.
Answer:
a) Yes.
Since the standard reduction potential of silver is more than that of copper it is less reactive than copper and hence cannot react with CuSO4.
b) Zinc with negative standard electrode potential is more active than hydrogen and hence can displace hydrogen from dil.H2SO4. But copper with a positive standard electrode potential is less active than hydrogen and hence cannot displace hydrogen from dil. H2SO4.
c) Since silver is less active than hydrogen, when silver electrode is connected with S.H.E, silver will act as cathode and S.H.E will act as anode. At the anode H is oxidiseci to H+. Hence, the reaction taking place at S.H.E is
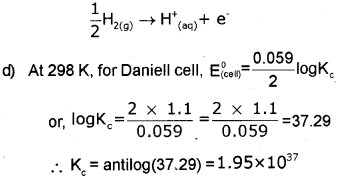
Question 5.
Daniell cell is a galvanic cell made of zinc and copper electrodes. (March – 2012)
i) Write anode and cathode reactions in Daniell cell.
ii) Nernst equation for the electrode reaction
Mn+ + + ne- 2 M is
\(\mathrm{E}_{\left(\mathrm{M}^{n+} / \mathrm{M}\right)}=\mathrm{E}_{\left(\mathrm{M}^{n+} / \mathrm{M}\right)}^{0}-\frac{2.303 \mathrm{RT}}{\mathrm{nF}} \log \frac{1}{\left[\mathrm{M}^{\mathrm{n}^{+}}\right]}\)
Derive Nernst equation for Daniel cell.
OR
Leclariche cell, Lead storage cell and Fuel cell are galvanic cells having different uses.
i) Among these, the Leclanche cell is a primary cell and Lead storage cell is a secondary cell. Write any two differences between primary cells and secondary cells.
ii) What is a Fuel cell?
iii) Write the overall cell reaction in H2 – O2 Fuel cell.
Answer:
i) Anode : Zn ? Zn2+ + 2e- (oxidation half reaction)
Cathode : Cu2+ + 2e– ? Cu (reduction half reaction)
Overall cell reaction is Zn + Cu2+ ? Zn2+ + Cu Theceilcanberepresentedas: Znl Zn2+llCu2+lCu
Anode : Zn I ZnSO4
Cathode : Cu I CuSO4
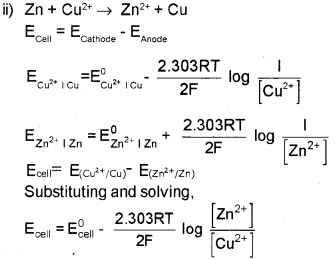
which is the Nernst equation for Daniell cell.
OR
| 1) Primary cell | Secondary cell |
| a) Electrode reaction cannot be reversed. | a) Electrode reaction can be reversed by an external electric energy source. |
| b) Reaction occur only once & after use they become dead; not chargeable. | b) Reaction can occur many times in both directions Rechargeable |
ii) Fuel cells are galvanic cells in which chemical energy from fuels like H2, CO, CH4 etc are converted to electrical energy.
Question 6.
Innumerable number of galvanic cells can be constructed on the pattern of Daniell cell by taking combination of different half cells. (Say – 2012)
i) What is galvanic cell?
ii) Name the anode and cathode of the Daniell cell.
iii) Write the name of the half-cell represented by Pt(S)/H2(g)/H+(aq).
iv) What is the potential of the above half-cell at all temperate res?
Answer:
i) It is a device for converting chemical energy released into electrical energy.
ii) Anode – Zn rod dipped in ZnSO4
Cathode – Cu rod dipped on CauSO4
iii) Standard Hydrogen Electrode (S.H.E)
iv) S.H.E is assigned a zero potential at all temperatures.
Question 7.
With decrease in concentration of an electrolytic solution, conductivity (K)decreases and molar conductivity (?m) increases. (March – 2013)
i) Write the equation showing the relationship between conductivity and molar conductivity.
ii) How will you account for the increase in molar conductivity with decrease in concentration?
iii) Limiting molar conductivity (L°m) of a strong electrolyte can be determined by graphical extrapolation method. Suggest a method for the determination of limiting molar conducivity of a weak electrolyte, taking acetic acid (CH3COOH) as example.
Answer:
i) Molar conductivity \(\left(\Lambda_{m}\right)=\frac{\kappa \times 1000}{M}\)
where M ? Molanty and K ? Conductivity
OR
\(\lambda_{\mathrm{m}}=\frac{\mathrm{K}}{\mathrm{C}}\) Concentration of where solution in mol/litre.
ii) Molar conductivity increase with decrease in con centration or increase in dilution as number of ions as well as mobility of ions increases with dilution.
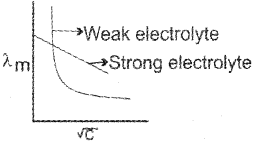
a) For strong electrolytes, the number of ions do not increase appreciably on dilution and only mobility of ions increases due to decrease in interionic attraction.
? increases a little as shown in the above graph.
b) For weak electrolytes, the number of ions, as well as mobility of ions, increases on dilution. Hence, increases steeply on dilution, especially near lower concentrations as shown in graph.
iii) Using Kohlrauschs law
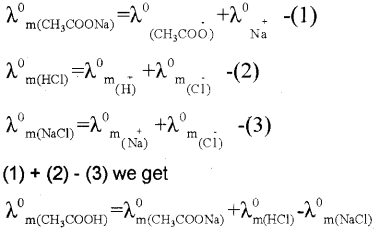
Thus the molar conductivity of CH3COQH at infinite dilution can be determined from the knowledge Of \(\lambda_{\mathrm{m}\left(\mathrm{CH}_{3} \mathrm{COONa}\right)}^{0}, \lambda_{\mathrm{m}(\mathrm{HCl})}^{0}, \lambda_{\mathrm{m}(\mathrm{NaCl})}^{0}\)
Question 8.
We can construct innumerable number of galvanic cells on the pattern of Daniell cell by taking combi nation of different half cells. (Say – 2013)
a) What is a galvanic cell?
b) Name the cathode and anode used in the Daniell cell.
c) Name the cell represented by Pt(S), H2(g)/H+(aq).
d) According to convention what is the potential of the above cell at all temperatures?
e) Write the use of the above cell.
Answer:
a) It is a device for converting chemical energy into electrical energy. The decrease in free energy in a spontaneous chemical process appears as elec trical energy. e.g., Daniell cell,
b) A zinc rod dipped in 1 M solution of ZnSO4 acts as the anode. Here oxidation takes place. A copper rod dipped in 1 M solution of CuSO4 acts as the cathode. Here reduction takes place.
c) This represents the andard Hydrogen Electrode (S.H.E), when it acts as the anode.
d) According to convention, S.H.E is assigned a zero potential at all temperatures
e) It is used as a primary reference electrode for determining the standard electrode potential of an unknown electrode. The electrode whose standard potential is to be determined is coupled with a reference electrode of known potential i.e., S.H.E to get a galvanic cell. The potential of the resulting galvanic cell is determined experimentally. E = E – E Knowing the potential of one electrode that of the other can be calculated.
Question 9.
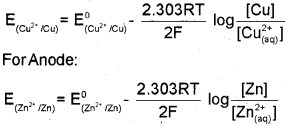
a) The cell reactìon in Daniell cell is Zn(s) + CU2+(aq) ? Zn2+(aq) + CU(s) and Nernst equation for single electrode potential for general electrode reaction \(\mathrm{M}^{\mathrm{n}+}{ }_{(\mathrm{aq})}+\mathrm{ne}^{-} \longrightarrow \mathrm{M}_{(\mathrm{s})}\) is \(E_{M^{n+} M}=E_{M^{n+} / M}^{0}-\frac{2.303 R T}{n F} \log \frac{[M]}{\left[M^{n+}\right]}\) Derive Nernst equation for Daniell cell. (March – 2014)
b) Daniell cell is a primary cell while lead storage cell is a secondary cell. Write any one difference between primary cells and secondary cells.
Answer:
a) In Daniell cell, the electrode potential for any given concentration of Cu2+ and Zn2+ ions, we can write, For Cathode:
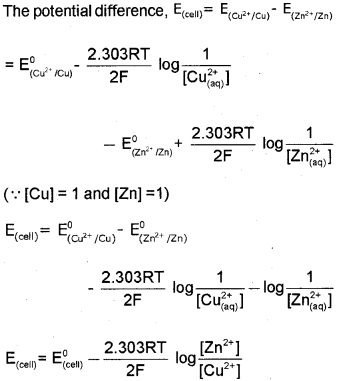
b) In primary cells the reaction occurs only once and after use over a period of time cell becomes dead and cannot be reused again. Here the cell reaction is irreversible. e.g., dry cell Secondary cells after use can be recharged by passing current through them in the opposite direction so that they can be used again. Here the cell reaction is reversible. e.g., Lead storage cell.
Question 10.
Fuel cells are special types of galvanic cells. (Say – 2014)
a) i) Whataregalvaniccells?
ii) Write any two advantages of fuel cells.
b) Write the electrode reactions is H2 – O2 fuel cells.
a) i) These are devices for converting chemical energy into electrical energy. The decrease in free energy in a spontaneous chemical process appears as electrical energy. e.g., Daniell oeil.
ii) 1) Fuel cells are pollution free.
2) Fuel cells are highly efficient (about 70%) compared to thermal plants (about 40%)
3) Fuel cells run continuously as long as the reactants are supplied. (any two)
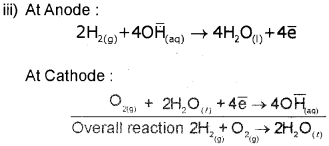
b) At cathode:
O2(g) + 2H2O(l) + 4e ? 4OH(aq)
At anode:
2H2(g) + 4OH(aq) ? 4H2O(l)
Question 11.
You are supplied with the following substances: Copper rod, Zinc rod, Salt bridge, two glass bea kers, a piece of wire, 1 M CuSO4 solution, 1 M ZnSO4 solution. (March – 2015)
a) Represent the cell made using the above materials.
b) i) Write the Nemst equation for the above cell.
ii) Calculate the standard EMF of the cell if
\(\begin{array}{l}
E_{\left(\mathrm{Zn}^{2+} \mid \mathrm{Zn}\right)}=-0.76 \mathrm{~V} \\
\mathrm{E}_{\left(\mathrm{Cu}^{2+} \mid \mathrm{Cu}\right)}=+0.34 \mathrm{~V}
\end{array}\)
Answer:
a) Zn(s)|Zn2+(1 M)||Cu2+(1 M)|Cu(s)
OR
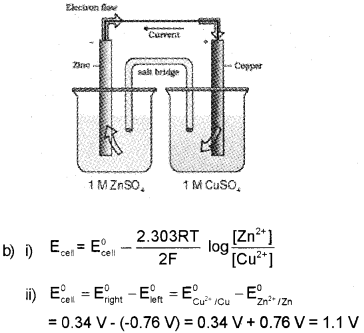
Question 12.
a) Conductance (G), conductivity (K) and molar conductivity (?m) are terms used in electrolytic conduction. (Say – 2015)
i) Write any two factors on which conductivity depends on.
ii) How do conductivity and molar conductivity vary with concentration of electrolytic solution?
b) Write any one difference between primary cell and secondary cell.
Answer:
a) i) 1. the nature of the electrolyte added
2. size of the ions produced and their solvation
3. the nature of the solvent and its viscosity
4. concentration of the electrolyte
5. temperature (any two factors)
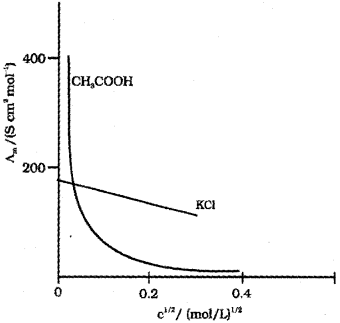
ii) Conductivity always decreases with decrease in concentration both for weak and strong electrolytes. This can be explained by the fact that the number of ions per unit volume that carry the current in a solution decreases on dilution.
Molar conductivity, ?m = kV
?m increases with decrease in concentration. This is because the total volume (V) of the solution containing one mole of electrolyte also increases. The decrease in K on dilution is more than compensated by increase in its volume.
In the case of strong electrolytes Am increases slowly with dilution and can be represented by the equation:
\(\Lambda_{m}=\Lambda_{m}^{0}-A c^{1 / 2}\)
where ‘c’ is the molar concentration, ‘A’ is a constant (equato to -ve of slope) and ?m° is the limiting molar conductivity. Here, the plot of ?m against ‘C1/2‘ will be a straight line. In the case of weak electrolytes ?m increases steeply on dilution, especially near lower concentrations due to increase in degree of dissociation.
b) Pnmary cell – Cell in which the reaction occurs only once and after use over a period of time the cell becomes dead and cannot be reused again. Secondary cell – Cell which can be recharged after use by passing current through it in the opposite direction so that it can be used again.
Question 13.
a) Which of the following is a secondary cell? (March – 2016)
a) Dry cell
b) Leclanche cell
C) Mercury cell
d) None of these
b) What is the relationship between resistance and conductance?
c) One of the fuel cells uses the reaction of hydrogen and oxygen to form water. Write down the cell re action taking place in the anode and cathode of that fuel cell.
Answer:
d) None of these
b) Resistance is inversely proportional to conductance. Or, Conductance is the inverse of resistance.
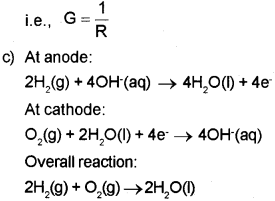
Question 14.
Galvanic cells are classified into primary and secondary cells (Say – 2016)
a) Write any two differences between primary cell and secondary cell.
b) i) What is a fuel cell?
ii) Write the overall cell reaction in H2 – O2 fuel cell.
Answer:
a) Primary cell
Cell reaction cannot be reversed and hence can not be recharged, cannot be reused again. e.g. Dry cell, Mercury cell
Secondary cell
Cell reaction can be reversed and hence can be recharged, can be resued again. e.g. Lead storage battery, nickel-cadmium cell
b) i) Fuel cell is a galvanic cell that is designed to convert the energy of combustion of fuels directly into electncal energy.
ii) 2H2(g) + O2(g) ? 2H2O(l)
Question 15.
a) Represent the galvanic cell based on the cell reaction given below (March – 2017)
\(\mathrm{Cu}_{(\mathrm{s})}+2 \mathrm{Ag}_{(\mathrm{aq})} \rightarrow \mathrm{Cu}_{(\mathrm{aq})}^{2+}+2 \mathrm{Ag}_{(\mathrm{s})}\)
b) Write the half cell reaction of the above cell.
c) ?m0 for NaCI. HCI and NaAc are 126.4, 425.9 and 91.0 S cm2 mol-1 respectively. Calculate ?m0 for HAc.
Answer:
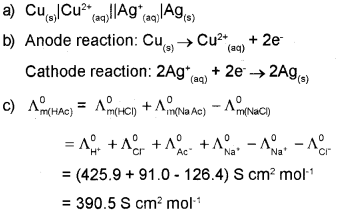
Question 16.
a) Identify the weak electrolyte from the following: (Say – 2017)
i) KCl
ii) NaCl
iii) KBr
iv) CH3COOH
b) Kohlrausch’s law helps to determine the degree of dissociation of a weak electrolyte at a given concentration.
i) State Kohlrausch’s law.
ii) The molar conductivity ?m of .001 M acetic acid is 4.95 x 10-5 S cm2 mol-1. Calculate the degree of dissociation (a) at this concentra tion if limiting molar conductivity \(\wedge_{m}^{0}\) for H+ is 340 x 10-5 S cm2 mol-1 and for CH3COO is 50.5 x 10-5 S cm2 mol-1.
Answer:
a) iv) CN3COOH
b) i) Kohlrausch’s law: Limiting molar conductivity of an electrolyte is the sum of individual contnbutions of anion and cation respectively.
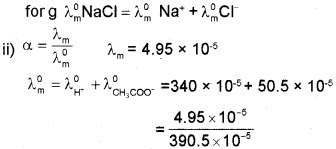
We hope the Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 3 Electrochemistry help you. If you have any query regarding Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 3 Electrochemistry, drop a comment below and we will get back to you at the earliest.
