Plus Two Chemistry Chapter Wise Previous Questions Chapter 2 Solutions is part of Kerala Plus Two Chemistry Chapter Wise Previous Year Questions and Answers. Here we have given Plus Two Chemistry Chapter Wise Questions and Answers Chapter 2 Solutions.
Kerala Plus Two Chemistry Chapter Wise Previous Questions Chapter 2 Solutions
Question 1
Colligative properties are properties of solutions which depend on the number of solute particles irrespective of their nature. (March – 2010)
a) Name the four important colligative properties.
b) What happens to the colligative properties when ethanoic acid is treated with benzene? Give reason.
Answer:
a) 1) Relating lowering of vapour pressure of the solvent
2) Depression of freezing point of the solvent
3) Elevation of Boiling point of the solvent
4) Osmotic pressure of the solution
b) Molecules of ethanoic add (acetic acid) dimenses in benzene due to hydrogen bonding. As a result of dimerisation, the actual number of solute particles in solution is decreased. As colligative property decreases molecular mass increases.
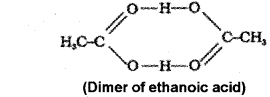
Question 2.
a) Mr. Raju has determined the molecular masses of different solutes in different solvents by osmotic pressure measurements and presented them in the following table. Please help him to complete the table. (Say – 2010)
| Solute | Solvent | Theoretical Molecular Mass | Experimental Molecular Mass |
| NaCI Benzoic acid Urea Acetic acid CaCI2 Glucose Ai2(So4)3 | Water Benzene Water Benzene Water Water Water | A B C D E F G | A/2 — — — — — — |
b) The extent of deviation from ideal behaviour of a solution is explained by van’t Hoff factor, i. What is meant by van’t Hoff factor?
Answer:
a) 2B, C, 2D, E/3, F, G/5.
b) 2B and 2 D are due to association
\(\mathrm{i}=\frac{\text { Normal molar mass }}{\text { Abnormal molar mass }}\)
OR
\(\mathrm{i}=\frac{\text { Observed colligative property }}{\text { Calculated colligative property }}\)
Question 3.
Colligative properties can be used to determine the molecular mass of solutes in solutions. (March – 2011)
a) What do you mean by ‘Colligative Property’?
b) Fordeterminingthe molecular mass of polymers, osmotic pressure is preferred to other properties. Why?
c) For intravenous injections only solutions with freezing point depression equal to that of 0.9% NaCI solution is used. Why?
Answer:
a) Colligative properties are those properties which depend upon no. of particles in the solution.
b) i) Pressure measurement can be done around the room temperature.
ii) Molarity of the solution is used instead of molality.
iii) Its magnitude is large compared to other colligative properties.
iv) Polymers have poor solubility.
c) This is because the osmotic pressure associated with the fulid inside the blood cell is equivalent to that of 0.9 (m/w) sodium chloride solution, i.e., blood is isotonic with this solution.
Question 4.
Relative lowering of vapour pressure, elevation of boiling point, depression of freezing point, and osmotic pressure are important colligative properties of dilute solutions. (Say – 2011)
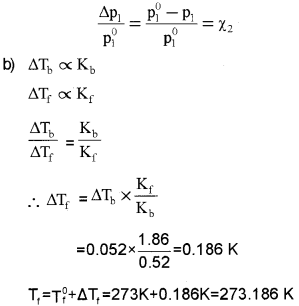
a) Relative lowering of vapour pressure of an aqueous dilute solution of glucose is 0.018. What is the mole fraction of glucose in the solution?
b) An aqueous dilute solution of a non-volatile solute boils at 373.052 K. Find the freezing point of the solution.
For water Kb = 0.52K Kg mol-1
For water Kf = 1.86 K Kgmol-1
Normal boiling point of water = 373K
Normal freezing point of water = 273K
Answer:
a) 0.018
Because according to Raoult’s law for solutions containing non-volatile solutes, the relaive lower-ing of vapour pressure is equal to the mole fraction of the non-volatile solute.
Question 5.
Vapour pressure of a solution is different from that of pure solvent. (March – 2012)
i) Name the law which helps us to determine partial vapour pressure of a volatile component in solution.
ii) State the above law.
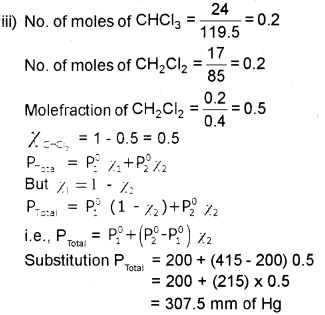
iii) Vapour pressure of chloroform (CHCI3) and dichloro methane (CH2CI2) at 298 K are 200 mm and 415 mm of Hg respectively. Calculate the vapour pressure of solution prepared by mixing 24 g of chloroform and 17 g of dichloro methane at 298 K. [At. Mass : H -1, C – 12, Cl – 35.5]
Answer:
i) Raoult’s law.
ii) It states that at a given temperature for a solution of volatile liquids, the partial v.p. of each component in the solution is directly proportional to its mole fraction.
Question 6.
Colligative properties are properties of solutions which depend on the number of solute particles in the solution. (Say – 2012)
i) Write the names of four important colligative properties.
ii) The value of van’t Hoff factor, ‘i’ for aqueous KCI solution is close to 2, while the value of ‘i’ for ethanoic acid in benzene is nearly 0.5. Give reason.
i) The Colligative Properties are:
1) Relative lowering of vapour pressure
2) Elevation of boiling point
3) Depression of freesing point
4) Osmotic pressure
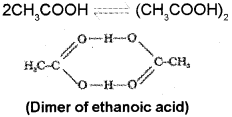
ii) This is caused by dissociation in the case of KCI and association in the case of acetic acid.
KCI in aqueous solution undergoes dissociation as KCI ? K+ + Cl–
Thus, if complete ionisation occurs the number of particles in solution becomes double and hence van’t Hoff factor (i) for aqueous KCI solution is close to 2.
In the case of ethanoic acid (acetic acid) association (dimerisation) occurs in benzene through in- termolecular hydrogen bonding. Thus, if complete association occurs the number of particles in solution becomes half and hence van’t Hoff factor (i) for ethanoic acid in benzene is nearly 0.5.
Question 7.
Elevation of boiling point is a colligative property, (March – 2013)
i) What are colligative properties?
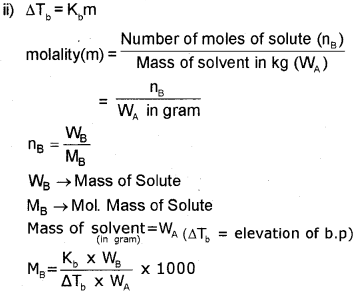
ii) Elevation of boiling point (D Tb) is directly proportional to molality (m) of solution.
Thus, DTb = Kbm, Kb is called the molal elevation constant.
From the above relation derive an expression to obtain molar mass of the solute,
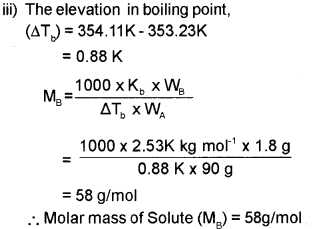
iii) The boiling point of benzene is 353.23K. When 1.80 g of a non-volatile solute was dissolved in 90g of benzene, the boiling point is raised to 354.11K. Calculate the molar mass of the solute. Kb for benzene is 2.53K kg mol-1.
Answer:
i) Colligative properties are those properties which depend upon the number of solute particles irrespective of their nature relative to the total number of particles present in the solution.
Question 8.
Liquid solutions can be classified into ideal and non-ideal solutions on the basis of Raoult’s law. (Say – 2013)
a) State Raoult’s law.
b) What are ideal solutions?
c) Write two important properties of ideal solutions.
d) What type of deviation is shown by a mixture of chloroform and acetone? Give reason.
Answer:
a) Raoult’s law states that for a solution of volatile liquids, the partial vapour of each component in the solution is directly proportional to its mole fraction. Or The partial vapour pressure of any volatile component of a solution is equal to the product of the vapour pressure of pure component and mole fraction of that component in the solution.
b) Ideal solutions are solutions which obey Raoult’s law over the entire range of concentration.
c) i) Enthalpy of mixing of the pure components to form the solution is zero, i.e.; ?mixH = 0
ii) Volume of mixing is zero, i.e.; ?mixV = 0
d) Negative deviation. This is because chloroform molecule is able to form hydrogen bond with acetone molecule.
Question 9.
Osmotic pressure is a colligative property and it is proportional to the molarity of the solution. (March – 2014)
a) What is osmotic pressure?
b) Molecular mass of NaCI determined by osmotic pressure measurement is found to be half of the actual value. Account for it.
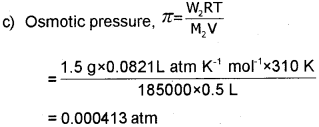
c) Calculate the osmotic pressure exerted by a solution prepared by dissolving 1.5 g of a polymer of molar mass 185000 in 500 mL of water at 37°C. [R = 0.0821 L atm K-1 mol-1].
Answer:
a) Osmotic pressure is the extra pressure applied on the solution to stop osmosis
b) NaCI, as a strong electrolyte dissociates to form Na+ and Ch ions. The number of particles doubles colligative property also doubles. Observed molecular mass will be half.
Question 10.
Molarity (M), molality (m) and mole fraction (x) are some methods for expressing concentrations of solutions. (Say – 2014)
a) Which of these are temperature independent?
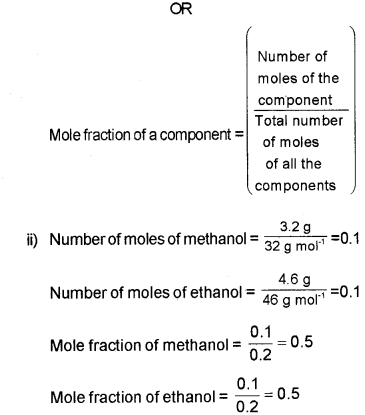
b) i) Define ‘molefraction’.
ii) A mixture contains 3.2 g methanol (molecular mass = 32 u) and 4.6 g ethanol, (molecular mass = 46 u) Find the molefraction of each ’ component)
Answer:
a) Molality and Mole fraction are temperature independent because these are mass to mass relationships. Mass is independent of temperature,
b) i) Mole fraction is defined as the ratio of the number of moles of that component to the total number of moles of all the components in solution.
Question 11.
a) Among the following which is not a colligative property? (March – 2015)
i) Osmotic pressure
ii) Elevation of boiling point
iii) Vapour pressure
iv) Depression of freezing point
b) i) 200 cm3 of an aqueous solution of a protein contains 1.26 g of protein. The osmotic pressure of solution at 300 K is found to be 8.3 x 10-2 bar. Calculate the molar mass of protein. (R = 0.083 L bar K-1 mol-1)
ii) What is the significance of van’t Hoff factor?
Answer:
a) iii) Vapour pressure
b) i) Osmotic pressure, n = 8.3 x 10-2 bar
Volume of the solution, V = 200 cm3 = 0.200 L
Temperature, T = 300 K
R = 0.083 L bar mol-1 K-1
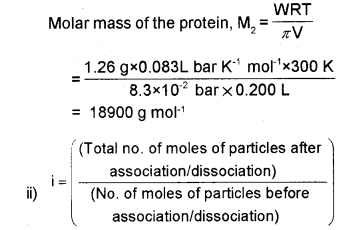
Thus, in the case of association, the value of ‘i’ is less than unity while for dissociation ‘i’ is greater than unity. If i =1 it means that there is no association or dissociation of the solute particles in solution.
Question 12.
a) Draw a vapour pressure curve, by plotting vapour pressure against mole fraction of an ideal solution of two volatile components A and B (not to scale). Indicate partial vapour pressure of A and B (PA and PB) and total vapour pressure (Ptotal) (Say – 2015)
b) What is an ideal solution?
c) Modify the above plot for non-ideal solution showing positive deviation. (Draw the above plot once again and modify).
Answer:
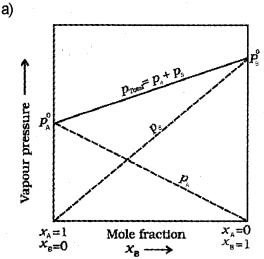
b) A solution which obeys Raoult’s law over the entire range of concentration is known as an ideal solution.
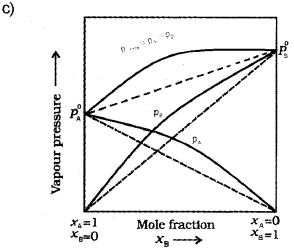
Question 13.
a) Number of moles of the solute per kilogram of the solvent is (March – 2016)
a) Mole fraction
b) Molality
c) Molarity
d) Molar mass
b) The extent to which a solute is dissociated or associated can be expressed by Van’t Hoff factor.’ Substantiate the statement.
c) The vapour pressure of pure benzene at a certain temperature is0.850 bar. Anon volatile, non-electrolyte solid weighing 0.5 g when added to 39 g of benzene (molar mass 78 g mol-1), the vapour pressure becomes 0.845 bar. What is the molar mass of the solid substance?
Answer:
a) b) Molality
b) b) The van’t Holf factor,

When i = 1 ? there is no association or dissociation of solute particles.
When i < 1 ? there is association of solute particles. When i > 1 ? there is dissociation of solute particles.
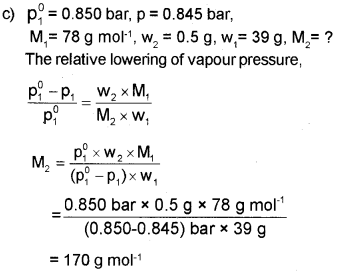
Question 15.
Osmotic pressure is a colligative property. (Say – 2016)
a) What is osmotic pressure?
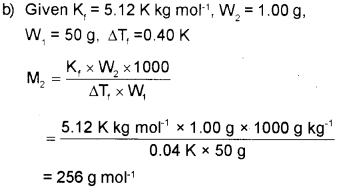
b) 1.00 g of a non-electrolyte solute dissolved in 50g of benzene lowered the freezing point of benzene by 0.40 k. The freezing point depression constant of benzene is 5.12 K kg/mol. Find the molar mass of solute.
Answer:
a) Osmotic pressure is the extra pressure applied on the solution side to just stop osmosis i.e., the flow of the solvent from its side to solution side through the semipermeable membrane,
Question 16.
a) Henry’s law is related to solubility of a gas in liquid. (March – 2017)
i) State Henry’s law.
ii) Write any two applications of Henry’s law.
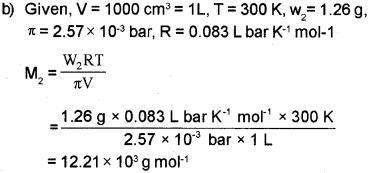
b) 1000cm3 of an aqueous solution of a protein contains 1.26 gm of the protein. The osmotic pressure of such a solution at 300 K is found to be 2.57 x 10-3 bar. Calculate molar mass of the protein. (R = 0.083 L bar mol-1 K-1)
Answer:
a) i) Henry’s law states that at constant tempera-ture, the solubility of a gas in a liquid is directly proportional to the pressure of the gas. p = KH x where, p is the partial pressure of the gas in vapour phase, KH is the Henry’s law constant and x is the mole fraction of the gas in the solution.
ii) 1. To increase the solubility of CO2 in soft drinks and soda water, the bottle is sealed under high pressure.
2. To avoid bends and the toxic effects of high concenration of nitrogen in blood, the tanks used by scuba divers are filled with air diluted with helium.
3. Low partial pressure of oxygen at high altitudes leads to low concentration of oxygen in the blood and tissues of people living at high altitudes or climbers and causes anoxia. (Any two applications)
Question 17.
a) The mole fraction of water in a mixture containing equal number of moles of water and ethanol is (Say – 2017)
i) 1
ii) 0.5
iii) 2
iv) 0.25
b) The following are the vapour pressure curves of a pure solvent and a solution of a non-volatile solute in it.
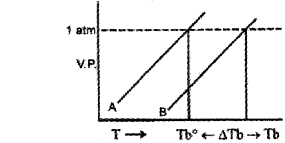
Based on the above curves answer the following questions:
i) What do the curves A and B indicate?
ii) Explain why the value of TB is greater than that of Tb0.
Answer:
a) ii) 0.5
b) i) A-Vapour pressure curve of solvent Vapour pressure curve of solution
ii) Due to the presence of a non-volatile solute vapour pressure of solution is less than solvent and the boiling point is increased.
We hope the Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 2 Solutions help you. If you have any query regarding Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 2 Solutions, drop a comment below and we will get back to you at the earliest.
