Plus Two Chemistry Chapter Wise Previous Questions Chapter 10 Haloalkanes and Haloarenes is part of Kerala Plus Two Chemistry Chapter Wise Previous Year Questions and Answers. Here we have given Plus Two Chemistry Chapter Wise Questions and Answers Chapter 10 Haloalkanes and Haloarenes.
Kerala Plus Two Chemistry Chapter Wise Previous Questions Chapter 10 Haloalkanes and Haloarenes
Question 1.
Most of the organic chlorides, bromides and iodides react with certain metals to give compounds containing carbon-metal bonds. (March – 2010)
i) Give one example for such a compound.
ii) How will you prepare the above compound?
b) Write any two electrophilic substitution reactions of chlorobenzene.
Answer:
i) Grignard reagent; CH3MgCI (Methyl magnesium chloride)
ii) Grignard reagents are prepared by treating haloalkanes with magnesium metal in dry ether.
CH3Cl+Mg dry ether ⟶CH3MgCl
b) Halogenation : When chlorobenzene is treated with chlorine in presence of anhydrous FeCl3 a mixture of 1,4-Dichlorobenzene (major product) and 1.2-Dichlorobenzene is formed.
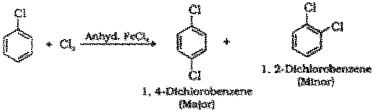
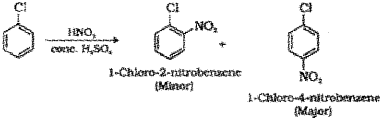
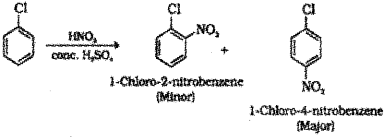
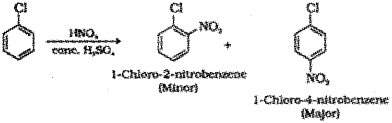
Nitration : When chlorobenzene is treated with nitrating mixture (mixture of conc.HNO3 and cone. H2SO4) a mixture of 1-Chloro-4-nitrobenzene (major product) and 1-Chloro-2-nitrobenzene (minor product) is formed.
Question 2.
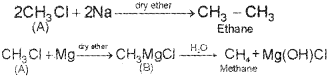
An organic compound A reacts with metallic sodium in ether medium to form ethane. A reacts with Magnesium in ether medium to give B, which on hydrolysis gives methane. Identify A and B. Write down the chemical equations for the reactions involved. (Say – 2010)
OR
Bromoethane, when treated with alcoholic KOH, gives ethene, KBr and H2O.
a) Identify the type of reaction.
b) Instead of bromoethane, if you take 2- bromobutane, what is the major product obtained? Write down the chemical equation for the reaction.
c) Explain the rule behind the above reaction.
Answer:
A → A CH3 Cl (Methyl chloride)
B → CH3Mg Cl (Methyl magnesium chloride)
OR
a) β – elimination (Dehydrohalogenation)
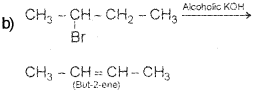
c) Saytzeffs rule – It states that in dehydro-halogenation reactions, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms.
Question 3.
Haloalkanes and Haloarenes react with metals to give Hydrocarbons or products from which hydrocarbons are obtained easily.
(March – 2011)
![]()
Identify the product and name the reaction.
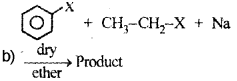
Identify the product and name the reaction.
![]()
Identify A & B.
Answer:
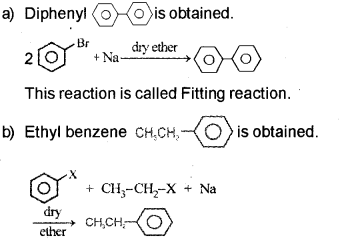
This reaction is called the Wurtz-Fitting reaction.
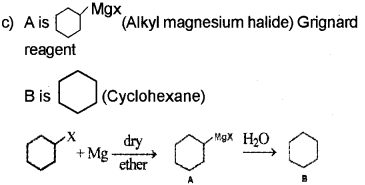
Question 4.
Alkyl halides are the starting materials for the synthesis of a number of organic compounds. How are the following compounds obtained from the alkyl halide CH3 – CH2 – Br? (Say – 2011)
a) Ethene
b) Ethanol
c) Butane
d) Ethoxy Ethane
Answer:
a) Conversion of bromoethane to ethene – By β – elimination reaction.
OR
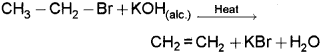
b) Conversion of bromoethane to ethanol – by alkaline hydrolysis.
When bromoethane is treated with aqueous KOH or moist Ag20 ethanol is formed.
OR
CH3−CH2−Br+KOH(aq)→CH3−CH2−OH+KBr
c) Conversion of bromoethane to n-butane – by Wurtz reaction.
When bromoethane is treated with sodium in dry ether n-Butane is formed.
OR
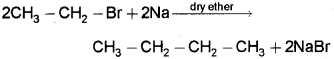
d) Conversion of bromoethane to ethoxyethane – by Williamson’s synthesis.
When bromoethane is treated with sodium ethoxide ethoxyethane is formed.
OR
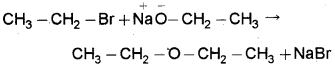
Question 5.
Nucleophilic substitution reactions are of two types – SN1 reactions and SN2 reactions. (March – 2012)
i) Write any two differences between SN1 and SN2 reactions.
ii) Write any two reasons for the less reactivity of aryl halides towards nucleophilic substitution reactions.
Answer:
i)
| SN1 reaction | Sn2 reaction |
| 1. Molecularity is 1. | 1. Molecularity is 2. |
| 2. Rate of reaction is dependent only on the concentration of the alkyl halide. | 2. Rate of reaction is dependent on the concentration of the alkyl halide as well as a nucleophile. |
| 3. Mechanism involves two steps – formation of carbocation followed by the nucleophilic attack. | 3. Mechanism involves one step via the formation of a transition state. |
| 4. Starting with an optically active alkyl halide results in partial racemization. | 4. Starting with an optically active alkyl halide results in a complete inversion of configuration. |
ii) Aryl halides are much less reactive than haloalkane or alkyl halides towards nucleophilic substitution reaction due to
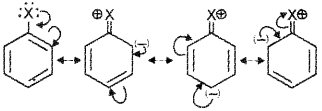
1) Resonance effect – in haloalkanes the electron pairs on halogen atom are in conjugation with -IT electrons of the ring and thus the C – X bond acquires a partial double bond character. Asa result the bond cleavage in haloarenes is difficult than in haloalkanes.
2) Difference in hybridization of carbon atom in C – X bond – in haloalkanes, the carbon atom attached to halogen is sp3 hybridized while in case of haloarenes the carbon atom attached to halogen is sp2 hybridized which is more electronegative. Hence, the C – X bond length in haloarenes (169 pm) is less than that in haloalkanes (177 pm). It is difficult to break a shorter bond than a longer bond. Therefore, Haloarenes are less reactive towards nucleophilic substitution reaction.
3) InstabilIty of phenyl cation – in case of haloarenes, the phenyl cation formed as a result of self-ionization will not be stabilized by resonance and therefore, SN1 mechanism Is ruled out.
4) Because of the possible repulsion, it is less likely for the electron-rich nucleophile to approach electron-rich arenes,
Question 6.
Haloarenes undergo electrophilic substitution race fans. Explain the Important electrophilic substitution reactions of chlorobenzene. (Write dn the chemical equation) (Say – 2012)
Answer:
Halogenation : When chlorobenzene Is treated with chlorine In presence of anhydrous FeCl3 a mixture of 1. 4-Dichlorobenzene (major product) and I 2-Dichlorobenzene b formed.
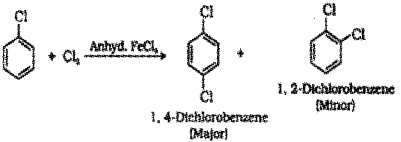
Nitration : When chlorobenzene is treated with nitrating mixture (mixture of conc.HNO3 and conc. H2SO4) a mixture of 1-Chloro-4-nitrobenzene (major product) and 1-Chloro-2-nitrobenzene (minor product) is formed.
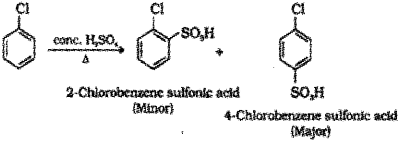
Sulphonation : When chlorobenzene is heated with conc.H2SO4 a mixture of 4-Chiorobenzene suiphonic acid (major product) and 2-Chioroben-zene suiphonic acid (minor product) is formed.
Question 7.
a) For the preparation of alkyl chlorides from alcohols, thionyl chloride (SOCl2) is preferred. Give reason. (March – 2013)
b) Halo alkanes undergo b – elimination reaction in presence of alcoholic potassium hydroxide.
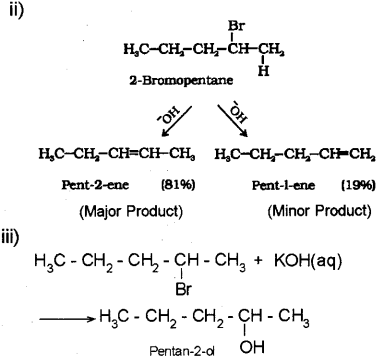
i) Which is the major product obtained by the b- elimination of 2-Bromo pentane?
ii) Name the rule, which leads to the product in the above elimination reaction.
c) Write the chemical equation for the preparation of toluene by Wurtz-Fitting reaction.
Answer:
a) S02 and HCI being escapable gases, the reaction of alcohols with thionyl chloride gives pure alkyl chlorides.
R-OH + SOCI2 → R-CI + SO2 + HCI
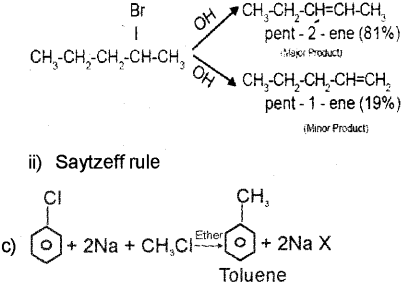
b) i) pent-2-ene is the major product since it has the greater number of alkyl groups attached to the doubly bonded carbon atoms.
Question 8.
Haloarenes undergo nucleophilic and electrophilic substitution reactions. (Say – 2013)
a) Write two examples for ambident nucleophiles.
b) Write one example for the nucleophilic substitution reaction of chlorobenzene.
c) Write any two examples of electrophilic substitution reaction of chlorobenzene.
Answer:
a) Cyanide ion (CN–) and nitrite ion (NO2).
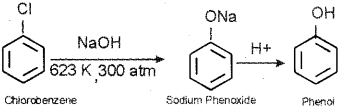
b) Chlorobenzene when heated with aqueous sodium hydroxide solution at a temperature of 623 K and a pressure of 300 atmosphere gets converted to phenol by a nucleophilic substitution reaction.
c) Nitration – When chloroform is treated with the nitrating mixture (a mixture of conc, HNO3 and cone. H2SO4), a mixture of 1-Chloro-2-nitrobenzene (minor product) and 1-Chloro-4-nitrobenzene (major product) is obtained.
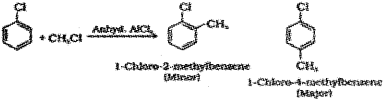
Friedel-Crafts alkylation – When chloroform is treated with methyl chloride in presence of anhydrous AICI3 a mixture of 1 – Chloro – 2 – methylbenzene (minor product) and 1-Chloro-4-methylbenzene (major product) are obtained.
Question 9.
a) Most important chemical reactions of haloalkanes are their substitution reactions. (March – 2014)
i) What is SN1 reaction?
ii) Arrange the four isomeric bromo butanes in the increasing order of their reactivity towards SN1 reaction.
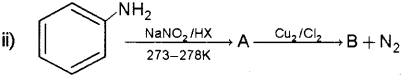
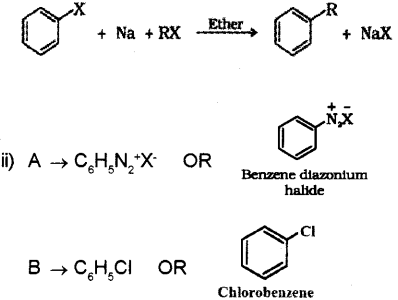
b) How will you prepare chlorobenzene from benzene diazonium chloride?
Answer:
a) i) SN1 reaction is a unimolecular nucleophilic substitution reaction. It involves the substitution of a weaker nucleophile by a stronger one and follows first-order kinetics, i.e., the rate of reaction depends upon the concentration of only one reactant. It occurs in two steps. In step I, the polarised C – X bond undergoes slow cleavage to produce a carbocation.
ii) SN1 reaction follows the order 10 alkyl halides < 2° alkyl halides < 3° alkyl halides.
CH3CH2CH2CH2Br < (CH3)2CHCH2Br < CH3CH2CH(Br)CH3 < (CH3)3CBr
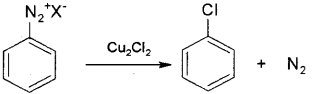
b) By Sandmeyer reaction – When freshly prepared benzene diazonium chloride solution is mixed with cuprous chloride the diazonium group is replaced by -Cl to form chlorobenzene.
OR
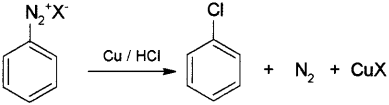
By Gatterman reaction – When freshly prepared benzene diazonium chloride solution is treated with hydrochloric acid in presence of copper pow- der the diazonium group is replaced by -Cl to form chlorobenzene.
Question 10.
a) i) Write ‘Saytzeff rule’. (Say – 2014)
ii) The products A and B of the following reaction are two isomeric alkenes. Identify Aand B.
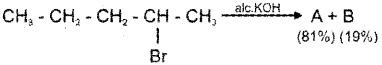
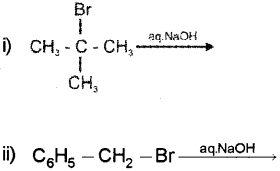
b) Identify the main product of the following reactions. Suggest whether the reaction is SN1 or SN2.
Answer:
a) i) The Saytzeff rule states that in dehydrohalogenation reactions, the preferred product is that alkene which has a greater number of alkyl groups attached to the doubly bonded carbon atoms.
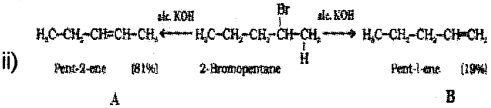
b) i) (CH3)3C−BraqNaOH⟶(CH3)3C−OH This reaction follows SN1 mechanism. Tertiary alkyl halides undergo SN1 reaction very fast because of the high stability of 3° carbocations.
ii) C6H5CH2Br⟶C6H5CH2OH
This reaction follows SN1 mechanism. Ben- zylic halides show high reactivity towards the SN1 reaction because the benzylic carbocation formed is stabilised through resonance.
Question 11.
a) Among the following which one is chlorine containing insecticide? (March – 2015)
i) DOT
ii) Freon
iii) Phosgene
iv) lodoform
b) Halo arenes undergo Wurtz-Fittig reaction.
i) What is Wurtz-Fittig reaction?
Write the formulae of A and B in the above reaction.
Answer:
a) i) DDT
b) i) A mixture of an alkyl halide and aryl halide gives an alkylarene when treated with sodium in dry ether.
Question 12.
i) State Saytzeff Rule. (Say – 2015)
ii) Identify the major and minor products obtained by the reaction between 2-bromo butane and alcoholic KOH.
iii) Write the product obtained by the reaction between 2-bromo butane and aqueous KOH.
iv) 2-bromo butane exhibit optical isomerism. What is optical isomerism?
Answer:
i) The Saytzeff rule states that in dehydro-halogenation reactions, the preferred product is that alkene which has a greater number of alkyl groups attached to the doubly bonded carbon atoms.
iv) Optical isomerism is the phenomenon in which molecules having the same molecular, as well as structural formulae, differ in the direction of rotation of the plane of plane polarised light.
Question 13.
a) Aryl halides are less reactive in nucleophilic substitution reactions. (March – 2016)
i) Write any two reasons for less reactivity,
ii) Give one example for nucleophilic substitution reactions of aryl halides.
b) Write a method for the preparation of alkyl halides.
c) Which of the following is not a polyhalogen compound?
a) Chloroform
b) Freon
c) Carbon tetrachloride
d) Chloro benzene
Answer:
i) 1. Resonance Effect- In aryl halides the electron pairs on halogen atom are in conjunction with π-electrons of the ring and different resonating structures are possible, e.g. resonance in chlorobenzene:
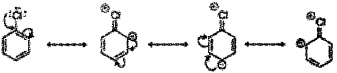
The C – X bond acquires a partial double bond character due to resonance. As a result, the bond cleavage in aryl halides is difficult than in alkyl halides and therefore, they are less reactive towards nucleophilic substitution reaction.
2. Difference in hybridisation of carbon atom in C-Xbond: In alkyl halides, the carbon atom attached to halogen is sp3 hybridised while in the case of aryl halides, the carbon atom attached to halogen is sp2 hybridised.
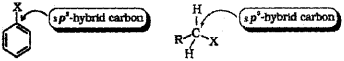
The sp2 hybridised carbon with a greater s-character is more electronegative and can hold the electron pair of C – X bond more tightly than sp3 hybridised carbon in alkyl halides with less s-character. Thus, the C – X bond length in aryl halides is less than that in alkyl halides. Since it is difficult to break a shorter bond than a longer bond, therefore, aryl halides are less reactive than alkyl halides towards nucleophilic substitution reaction.
3. Instability of phenyl cation – In the case of aryl halides, the phenyl cation formed as a result of self-ionization will not be stabilised by resonance and therefore, SN1 mechanism is ruled out.
4. Because of the possible repulsion, it is less likely for the electron-rich nucleophile to approach electron-rich arenes. (any two reasons)
ii) Chlorobenzene when heated in aqueous sodium hydroxide solution at a temperature of 623 K and a pressure of 300 atmospheres followed by acidification gets converted to phenol.
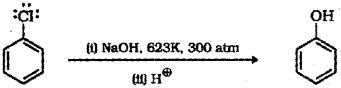
b) Alkyl halides can be prepared by treating alcohols with concentrated halogen acid in presence of anhydrous zinc chloride as a catalyst.
c) d) Chlorobenzene
Question 14.
Haloalkanes and haloarenes are compounds containing halogen atom. They undergo many types of reactions. (Say – 2016)
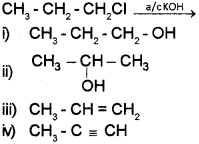
a) Identify the product formed in the following reaction:
b) i) Chloroform is stored in closed, dark coloured bottles completely filled up to the neck. Give reason.
ii) Write any two differences between SN1 and SN2 reactions.
Answer:
a) iii) CH3 – CH2 = CH2
b) i) Chloroform is slowly oxidised by air in the presence of light to an extremely poisonous gas, carbonyl chloride, also known as phosgene. It is therefore stored in closed dark coloured bottles completely filled so that air is kept out.
2CHCl3+O2 light ⟶2COCl2+2HCl
ii) Refer March 2012, Question 1 .(i)
Question 15.
a) An ambident nucleophile is …………. (March – 2017)
i) Ammonia
ii) Ammonium ion
iii) Chloride ion
iv) Nitrite ion
b) Halo alkanes and Halo arenes are organohalogen compounds.
i) Suggest a method for the preparation of alkyl chloride.
ii) Aryl halides are less reactive towards Nucleophilic substitution reactions.
Give reasons.
Answer:
a) iv) Nitrite ion
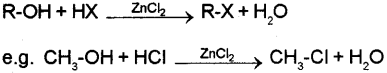
b) i) Alkyl chlorides can be prepared by passing dry hydrogen chloride gas through a solution of alcohol or by heating a solution of alcohol in concentrated aqueous HCI in presence of anhydrous zinc chloride as catalyst.
R−OH+HClZnCl2⟶R−Cl+H2O
![]()
Or, by the action of alcohols with PCl3, PCI5 or SOCI2.
3R-OH + PCl3 → 3R-CI + H3PO3
R-OH + PCl5 → R-CI + POCl3 + HCl
R-OH + SOCl2 → R-CI + SO2 + HCl
Or, chlorination of hydrocarbons in presence of light or heat.
eg. CH3CH2CH2CH3Cl2 NV ligtt or heat ⟶CH3CH2CH2CH2Cl+CH3CH2CH(Cl)CH3
Or, by the addition of hydrogen chloride to alkenes.
eg. CH2=CH2+HCl→CH3−CH3 (Any one method)
ii) Refer March 2012 Question 1 (ii)
Question 16.
On kinetic consideration, nucleophilic substitution in aryl/alkyl halides may be SN1 or SN2 mechanisms. (Say – 2017)
a) Briefly explain SN2 mechanism with an example.
b) In dehydrohalogenation of 2-Bromopentane why Pent-2-ene is major product and Pent-ene is minor product.
Answer:
a) SN2 mechanism (bimolecular nucleophilic substitution)
1. Takes place in a single step
2. Through the formation of the intermediate transition state
3. Inversion of configuration occurs
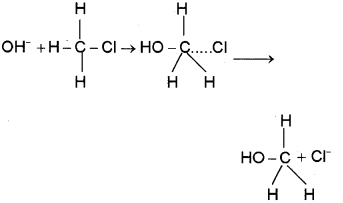
b) Saytzeff Rule: In dehydrohalogenation reaction, the more substituted alkene is the major product.
We hope the Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 10 Haloalkanes and Haloarenes help you. If you have any query regarding Kerala Plus Two Chemistry Chapter Wise Questions and Answers Chapter 10 Haloalkanes and Haloarenes, drop a comment below and we will get back to you at the earliest.
