Kerala Plus One Zoology Notes Chapter 4 Biomolecules
How To Analyse Chemical Composition?
Take any living tissue (a vegetable or a piece of liver, etc.) and grind it in trichloroacetic acid (Cl3CCOOH) using a mortar and a pestle. A thick slurry is formed. Then it is passed through a cheesecloth or cotton getting two fractions.
- Acid soluble fraction (Filtrate)
- Acid-insoluble fraction.
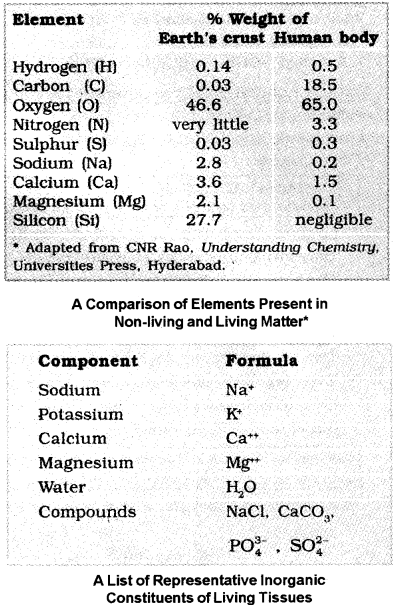
- All the carbon compounds from living tissues are called ‘biomolecules’.
- The tissue is fully burnt, all the carbon compounds are oxidised to gaseous form (C02, water vapour) and are removed.
- The remaining is called ‘ash’. It contains inorganic elements (like calcium, magnesium etc).
- Inorganic compounds like sulphate, phosphate, etc., are also seen in the acid-soluble fraction.
Organic compounds under biological view are classified into
- Amino acids:
- Nucleotide bases
- Fatty acids etc.
Amino acids:
- They are organic compounds containing four substituent groups occupying the four valency positions.
- These are hydrogen, carboxyl group, amino group and a variable group designated as R group.
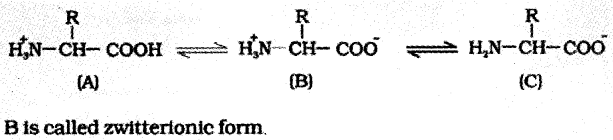
Based on the nature of the R group there are many amino acids. However, those which occur in proteins are only of twenty-one types.
The R group maybe
- Hydrogen (the amino acid is called glycine)
- A methyl group (alanine)
- Hydroxyl methyl (serine), etc.
Based on number of amino and carboxyl groups, there are
| 1. Acidic (eg: glutamic acid) 2. Basic (lysine) and neutral (valine) amino acids 3. Aromatic amino acids (tyrosine, phenylalanine, tryptophan) |
A particular property of amino acids is due to ionizable nature of —NH2 and —COOH groups
Fatty acid:
It has a carboxyl group attached to an R group.
The R group could be
- A methyl (—CH2)
- Ethyl (—C2H5)
Carbon number varies in different fatty acids:
- Palmitic acid – 16 carbon atoms
- Arachidonic acid – 20 carbon atoms
Fatty acids are
| 1. saturated (without double bond) 2. unsaturated (with one or more C = C double bonds) |
- Lipids possess both glycerol and fatty acids.
- They are monoglycerides, or diglycerides or triglycerides.
- These are also called fats and oils based on melting point. Oils have lower melting point eg: gingely oil.
- Some lipids have phosphorous, they are called phospholipids. They are found in cell membrane. eg: lecithin
Nitrogen bases:
- They are (heterocyclic rings) adenine, guanine, cytosine, uracil, and thymine
- If they are found attached to a sugar, they are called nucleosides.
- If a phosphate group is found esterified to the sugar, they are called nucleotides.
- Nucleic acids like DNA and RNA consist of nucleotides only.
| Adenosine, guanosine, thymidine, uridine and cytidine are nucleosides. Adenylic acid, thymidylic acid, guanylic acid, uridylic acid and cytidylic acid are nucleotides. |
Diagrammatic representation of small molecular weight organic compounds in living tissues.
Primary And Secondary Metabolites:
Primary metabolites:
Organic compounds such as amino acids, sugars, etc.are belongs to primary metabolites. Primary metabolites play important role in normal physiologial processes.
Secondary metabolites:
When analyse plant, fungal and microbial cells the alkaloides, flavonoides, rubber, essential oils, antibiotics, coloured pigments, scents, gums, spices etc are found. These are called secondary metabolites. Many of them are useful to ‘human welfare’ (eg: rubber, drugs, spices, scents and pigments).
| Pigments | Carotenoids, Ant.hocyanins, etc. |
| Alkaloids | Morphine, Codeine, etc. |
| Terpenoides | Monoterpenes, Diterpenes etc. |
| Essential oils | Lemon grass oil, etc. |
| Toxins | Abrin, Ricin |
| Lectins | Concanavalin A |
| Drugs | Vinblastin, curcumin, etc. |
| Polymeric substances | Rubber, gums, cellulose |
Biomacromolecules:
The acid insoluble fraction, has only four types of organic compounds i.e., proteins, nucleic acids, polysaccharides and lipids. These compounds, except lipids, have molecularweights in the range often thousand daltons and above.
Lipids, whose molecularweights do not exceed 800 Da, come under acid insoluble fraction. Hence Lipids are not macromolecules.
Biomicromolecules and biomacromolecules:
Molecular weights less than one thousand dalton are referred to as micromolecules or simply biomolecules while those which are found in the acid insoluble fraction are called macromolecules or biomacromolecules.
| Component | % of the total cellular mass |
| Water | 70 – 90 |
| Proteins | 10 – 15 |
| Carbohydrates | 3 |
| Lipids | 2 |
| Nucleie acid | 5 – 7 |
| Ions | 1 |
Proteins:
Proteins (heteropolymer)are linear chains of amino acids linked by peptide bonds i.e polymer of amino acids There are 21 types of amino acids (eg: alanine, cysteine, proline, tryptophan, lysine, etc.)
Some Proteins and their Function:
- Dietary proteins are the source of essential amino acids.
- Therefore, amino acids are essential or non-essential.
- Essential amino acids obtained through food.
Proteins carry out many functions in living organisms:
- some transport nutrients across cell membrane
- some fight infectious organisms
- Collagen is the most abundant protein in animal world and
- Ribulose bisphosphate Carboxylase – Oxygenase (RUBISCO) is the most abundant protein in the biosphere.
| Protein | Functions |
| Collagen | Intercellular ground substance |
| Trypsin | Enzyme |
| Insulin | Hormone |
| Antibody | Fights infectious agents |
| Receptor | Sensory reception (smell, taste, hormone, etc.) |
| GLUT-4 | Enables glucose transport into cells |
POLYSACCHARIDES
1. Polysaccharides are long chains of sugars.
2. Forexamplecellulose(homopolymer)is a polysaccharide consist of only one type of monosaccharide i.e. glucose.
3. Starch is storehouse of energy in plant tissues but animals have glycogen as energy source.
4. Inulin is a polymer of fructose.
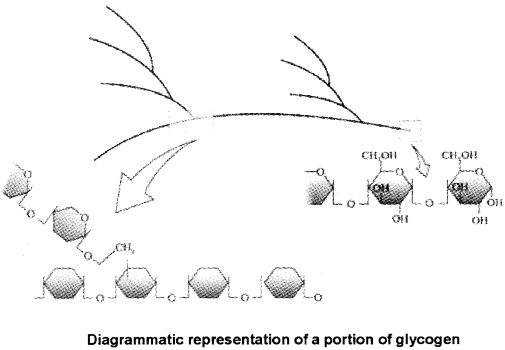
5. In a polysaccharide eg glycogen, the right end is called the reducing end and the left end is called the nonreducing end.
Starch forms helical secondary structures:
- Starch can hold l2 molecules in the helical portion. This reaction product blue in colour.
- Cellulose does not contain complex helices and hence cannot hold l2.
- Cotton fibre is cellulose
- The complex polysaccharides have as building blocks such as amino-sugars (eg: glucosamine, N— acetyl galactosamine, etc.).
- Exoskeletons of arthropods have a complex polysaccharide called chitin (heteropolymers)
Nucleic Acids:
Nucleic acids are another macromolecule found in the acid-insoluble fraction of living tissues. For nucleic acids, the building block is a nucleotide.
Components of nucleic acid:
- Heterocyclic compound(adenine, guanine, uracil, cytosine and thymine).
- Monosaccharide and
- Phosphoric acid or phosphate.
The sugar found in polynucleotides is either ribose (a monosaccharide pentose) or 2’ deoxyribose.
Nature of pentose sugar in DNA and RNA:
A nucleic acid containing deoxyribose is called deoxyribonucleic acid (DNA) while that which contains ribose is called ribonucleic acid (RNA).
Structure Of Proteins (Proteins are heteropolymers containing many amino acids):
Primary structure:
The sequence of amino acid in which the left end represented by the first amino acid (N— terminal amino acid )the right end represented by the last amino acid (C— terminal amino acid). This sequence forms linear structure. It is called the primary structure.
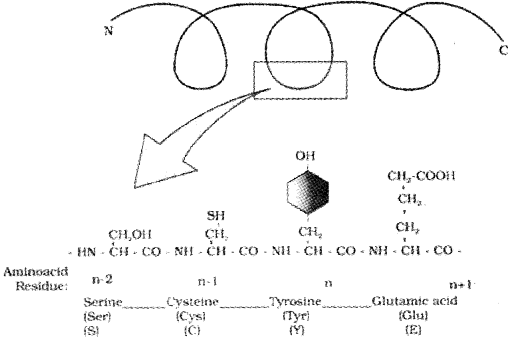
Primary structure of a portion of a hypothetical protein. N and C refer to the two termini of every protein. Single letter codes and three-letter abbreviations for amino acids are also indicated.
Secondary structure:
The primary structure has a rigid rod-like appearance which is folded in the form of a helix (similar to a revolving staircase). It appears as right-handed helices. It is called the secondary structure. Secondary structures exhibited by DNA is the Watson-Crick model. In this DNA exists as a double helix.
Tertiary structure:
The long protein chain is also folded upon itself like a hollow wollen ball, it called the tertiary structure. This gives us a 3-dimensional view of a protein. Tertiary structure is necessary for the many biological activities of proteins.
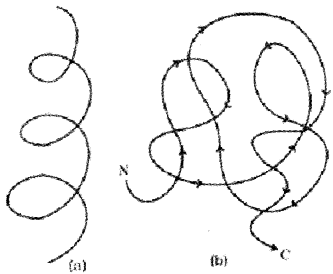
Quaternary structure:
Some proteins are assembled by more than one polypeptide chain. This is called the quaternary structure Adult human haemoglobin consists of 4 subunits. Two of these are identical to each other. Hence, two subunits are of a type and two subunits are of p type together constitute the human hemoglobin (Hb).
Nature Of bond linking Monomers In A Polymer:
1. Peptide bond:
In a protein, amino acids are linked by a peptide bond which is formed when the carboxyl (—COOH) group of one amino acid reacts with the amino (-NH2) group of the next amino acid with the elimination of a water.
2. Glvcosidic bond:
In a polysaccharide the individual monosaccharides are linked by a glycosidic bond. This bond is also formed by dehydration.
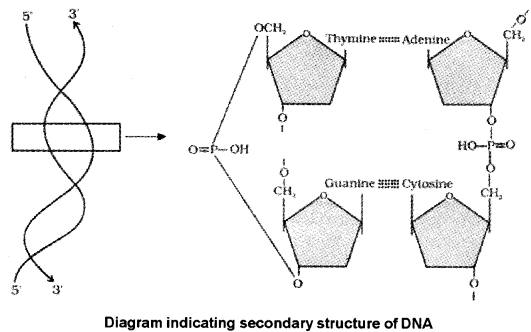
3. Phosphodiester Bond:
In a nucleic acid a phosphate moiety links the 3′-carbon of one sugar of one nucleotide to the 5′-carbon of the sugar of the succeeding nucleotide. The bond between the phosphate and hydroxyl group of sugar is called phosphodiester bond
DNA Structure:
- The two strands of polynucleotides are antiparallel i.e., run in the opposite direction.
- The backbone is formed by the sugar-phosphate-sugar chain.
- The nitrogen bases are A and G of one strand base pairs with T and C, respectively
- There are two hydrogen bonds between A and T but three hydrogen bonds are present between G and C.
- Each strand appears like a helical staircase.
- At each step of ascent, the strand turns 36°.
- One full turn of the helical strand have ten steps or ten base pairs.
- The pitch is 34A°. The distance between each base pairs is 3.4A°.
- This form of DNA is called B-DNA.
Dynamic State Of Body Constituents – Concept Of Metabolism:
Biomolecules are constantly being changed into some other biomolecules and also made from some other biomolecules. This is called turn over. This breaking and making is through chemical reactions constantly occurring in living organisms called as metabolism.
Metabolic reactions and transformation of biomolecules:
- removal of CO2 from amino acids making an amino acid into an amine,
- removal of amino group in a nucleotide base and
- hydrolysis of a glycosidic bond in a disaccharide
- Majority of these metabolic reactions are always linked to some other reactions. This series of linked reactions called metabolic pathways.
- These metabolic pathways are similar to the automobile traffic in a city.
- Another feature of these metabolic reactions is that every chemical reaction is a catalysed reaction.
- The catalysts which hasten the rate of a given metabolic conversation are also proteins. These proteins with catalytic power are named enzymes.
Metabolic Basis For Living:
- Metabolic pathways involves two processes The synthesis step is called anabolic pathways. The degradation step is called catabolic pathways.
- Catabolic pathways lead to the release of energy.
- For example, when glucose is degraded to lactic acid in our skeletal muscle, energy is liberated which stored in the form of chemical bonds, when needed, this bond energy is utilized.
Which is the energy currency of a cell?
- The energy currency in living systems is the bond energy in a chemical called adenosine triphosphate (ATP).
The Living State:
- All living organisms exist in a steady-state characterised by concentrations of each of these biomolecules.
- These biomolecules are in a metabolic flux. Any chemical or physical process moves spontaneously to equilibrium.
- The steady state is a non-equilibirium state. The systems at equilibrium cannot perform work. As living organisms work continuously, they cannot afford to reach equilibrium.
| Hence the living state is a non-equilibrium steady-state to be able to perform work. |
Metabolism provides a mechanism for the production of energy. Hence the living state and metabolism are synonymous. Without metabolism there cannot be a living state.
Enzymes:
| Almost all enzymes are proteins. Some nucleic acids that behave like enzymes are called ribozymes |
Enzvme activity:
- The tertiary structure is biologically active, an active site of an enzyme is a crevice or pocket into which the substrate fits.
- Thus enzymes, through their active site, catalyse reactions at a high rate.
- Enzymes are damaged at high temperatures (say above 40°C).
- Some enzymes isolated from organisms who normally live under extremely high temperatures (eg: hot vents and sulphur springs), are stable and retain their catalytic power even at high temperatures (upto 80° – 90°C).
- Thermal stability is thus an important quality of such enzymes isolated from thermophilic organisms.
Chemical Reactions:
Chemical compounds undergo two types of changes.
1. Physical change:
It involves the change in shape without breaking of bonds. eg: when ice melts into water, or when water becomes a vapour.
2. Chemical reaction/change:
When bonds are broken and new bonds are formed during transformation, this will be called a chemical reaction.Eg. Hydrolysis of starch into glucose is an organic chemical reaction. Rate of a physical or chemical process refers to the amount of product formed per unit time.
Role of enzvme in the rate of chemical reaction:
In the absence of any enzyme this reaction is very slow, with about 200 molecules of H2CO3 being formed in an hour. But using an carbonic anhydrase, the reaction speeds dramatically with about 600,000 molecules being formed every second.
The enzyme has accelerated the reaction rate by about 10 million times. A multistep chemical reaction, when each of the steps is catalysed by the same enzyme complex or different enzymes, is called a metabolic pathway.
- In glycolysis glucose becomes pyruvic acid through ten different enzyme catalysed metabolic reactions.
- Under normal aerobic conditions, pyruvic acid is formed.
- In yeast, during fermentation, the same pathway leads to the production of ethanol (alcohol).
- In our skeletal muscle, under anaerobic conditions, lactic acid is formed.
How do Enzymes bring about High Rates of Chemical Conversions?
The chemical which is converted into a product is called a ‘substrate’. Hence enzymes, i.e. proteins with three dimensional structures including an ‘active site’ convert a substrate (S) into a product (P).
What is the transition state?
During the state where substrate is bound to the enzyme active site, a new structure of the substrate called unstable transition state is formed. Then the bond breaking/making is completed, the product is released from the active site. The y-axis represents the potential energy content.
The x-axis represents the progression of the structural transformation or states through the ‘transition state’. If ‘P’ is at a lower level than ‘S’, the reaction is an exothermic reaction one need not to supply energy (by heating) in order to form the product.
However, whether it is an exothermic or spontaneous reaction or an endothermic or energy requiring reaction, the ‘S’ has to go through a much higher energy state or transition state. The difference in average energy content of ‘S’ from that of this transition state is called ‘activation energy’.
Enzymes bring down energy barrier making the transition of ‘S’ to ‘P’ more easy. Catalysed reactions proceed at rates faster than that of uncatalysed ones.
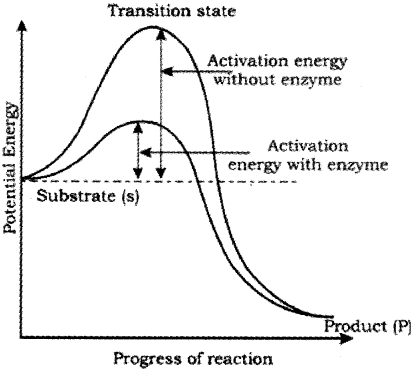
Nature of Enzyme Action:
![]()
Each enzyme (E) has a substrate (S) binding site in its molecule so that a highly reactive enzyme-substrate complex (ES) is produced. This complex is short-lived and dissociates into its products.
The catalytic cycle of an enzyme action can be described in the following steps:
| 1. First, the substrate binds to the active site of the enzyme. 2. The binding of the substrate induces the enzyme to alter its shape. 3. The active site of the enzyme, now in close proximity of the substrate breaks the chemical bonds of the substrate and the new enzyme- product complex is formed. 4. The enzyme releases the products of the reaction and the free enzyme is ready to bind to another molecule of the substrate. |
Factors Affecting Enzyme Activity:
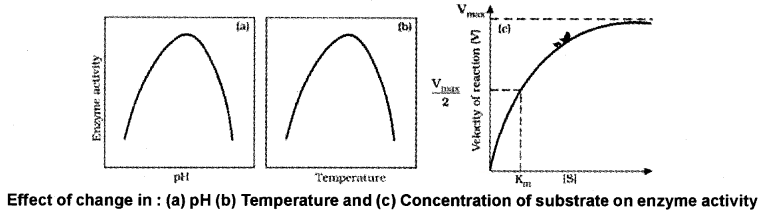
The activity of an enzyme can be affected by temperature, pH, change in substrate concentration.
1. Temperature and pH:
Each enzyme shows its highest activity at a particular temperature and pH called the optimum temperature and optimum pH. Low temperature preserves the enzyme in a temporarily inactive state whereas high temperature destroys enzymatic activity because proteins are denatured by heat.
2. Concentration of Substrate:
With the increase in substrate concentration, the velocity of the enzymatic reaction rises at first. The reaction ultimately reaches a maximum velocity (Vmax) which is not increased by further rise in concentration of the substrate because the enzyme molecules are saturated there are no free enzyme molecules to bind with the additional substrate molecules
Enzyme inhibition:
When the binding of the chemical shuts off enzyme activity, the process is called inhibition and the chemical is called an inhibitor. When the inhibitor closely resembles the substrate in its molecular structure and inhibits the activity of the enzyme, it is known as competitive inhibitor. eg: Inhibition of succinic dehydrogenase by malonate which closely resembles the substrate succinate in structure. Such competitive inhibitors are often used in the control of bacterial pathogens.
Classification and Nomenclature of Enzymes:
Enzymes are divided into 6 classes.
1. Oxidoreductases/dehydrogenases:
Enzymes which catalyse oxidoreduction between two substrates S and S’ eg:
![]()
2. Transferases:
Enzymes catalysing a transfer of a group, G (other than hydrogen) between a pair of substrate S and S’ eg:
\(\mathbf{S}-\mathbf{G}+\mathbf{S}^{‘} \longrightarrow \mathbf{S}+\mathbf{S}^{‘}-\mathbf{G}\)
3. Hydrolases:
Enzymes catalysing hydrolysis of ester, ether, peptide, glycosidic, C – C, C – halide or P – N bonds.
4. Lyases:
Enzymes that catalyse removal of groups from substrates by mechanisms other than hydrolysis leaving double bonds.
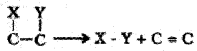
5. Isomerases:
Includes all enzymes catalysing inter-conversion of optical, geometric or positional isomers.
6. Lyases:
Enzymes catalysing the linking together of 2 compounds, eg: enzymes which catalyse joining of C – O, C – S, C – N, P – O etc. bonds.
Co-factors:
Enzymes are composed of one or several polypeptide chains and non-protein constituents called cofactors. They are bound to the enzyme to make the enzyme catalytically active. The protein part of the enzymes is called the apoenzyme.
Three kinds of cofactors are
- prosthetic groups
- co-enzymes
- Metal ions.
1. Prosthetic groups:
They are organic compounds that are tightly bound to the apoenzyme. For example, in peroxidase and catalase, which catalyze the breakdown of hydrogen peroxide to water and oxygen. Haem is the prosthetic group and it is a part of the active site of the enzyme.
2. Co-enzymes:
They are also organic compounds loosely bound to apoenzyme for catalysis. Co-enzymes serve as co-factors in a number of different enzyme-catalyzed reactions. Many coenzymes are vitamins eg: coenzyme nicotinamide adenine dinucleotide (NAD) and NADP contain the vitamin niacin.
2. Metations:
Zinc is a cofactor for the proteolytic enzyme carboxypeptidase. Catalytic activity is lost when the co-factor is removed from the enzyme.
