Kerala Plus One Chemistry Previous Year Question Paper March 2018 with Answers
| Board | SCERT |
| Class | Plus One |
| Subject | Chemistry |
| Category | Plus One Previous Year Question Papers |
Time Allowed: 2 hours
Cool off time: 15 Minutes
Maximum Marks: 60
General Instructions to Candidates:
- There is a ‘cool off time’ of 15 minutes in addition to the writing time.
- Use the ‘cool off time’ to get familiar with questions and to plan your answers.
- Read the instructions carefully.
- Read questions carefully before answering.
- Calculations, figures and graphs should be shown in the answer sheet itself.
- Malayalam version of the questions is also provided.
- Give equations wherever necessary.
- Electronic devices except non programmable calculators are not allowed in the Examination Hall.
Answer all questions form question numbers 1 to 7. Each carry 1 score. (Scores: 7 × 1 = 7)
Question 1.
Name the type of smog generally formed during cool and humid climate.
Answer:
Classical smog OR Reducing smog OR London smog
Question 2.
Predict the shape of XeF4 molecule, according to VSEPR theory.
Answer:
Square planar
or
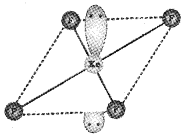
Question 3.
Which is the acidic oxide among the following?
a) Cl2O7
b) Na2O
c) Al2O3
d) CO
Answer:
a) Cl2O7
Question 4.
How many angular nodes are present in a 5f-orbitals?
Answer:
3
Question 5.
The allotrope of carbon with the highest thermodynamic stability is ………….
Answer:
Graphite
Question 6.
The critical temperatures of some gases are given in the following table:
| Gas | H2 | He | O2 | N2 |
| Critical temperature (K) | 33.2 | 5.3 | 154.3 | 126 |
If the samples of the above given gases are cooled from 298 K, which one will liquify first by applying pressure?
Answer:
O2
Question 7.
The number of oxygen atoms present in 5 moles of glucose (C6H12O6) is …………
Answer:
5 × 6 × NA = 30 × NA = 30 moles = 30 × 6.022 × 1023
= 1.8066 × 1025
Answer any ten from question numbers 8 to 20. Each carries two scores. (Scores: 10 × 2 = 20)
Question 8.
By using the concept of hybridization, explain the structure of H2O molecule.
Answer:
In the case of H2O molecule four orbitals of oxygen atom (one 2s and three 2p) undergo sp3 hybridisation of which two contain one electron each and the other two contain a pair of electrons. These four sp3 hybrid orbitals acquire a tetrahedral geometry, with two comers occupied by hydrogen atoms while the other two by the lone pairs. The bond angle is reduced to 104.5° from 109.5° and the molecule thus acquires a V-shape or angular geometry.
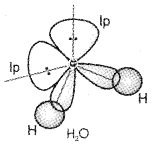
Question 9.
Give the IUPAC names of the following compounds.
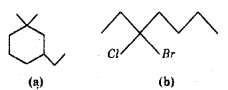
Answer:
a) 3-Ethyl-3-propylcyclohexane
b) 3-Bromo-3-chloroheptane
Question 10.
Write any two applications of green chemistry in day-to-day life.
Answer:
i) Dry cleaning of clothes – The process using tetrachloroethene is now being replaced by a process, where liquefied CO2 with a suitable detergent is used which will result in less harm to ground water.
ii) Bleaching of paper – Instead of Cl2 gas, hydrogen peroxide with suitable catalyst is used.
iii) Synthesis of chemicals – Ethanal is commercially prepared by one step oxidation of ethene in the presence of ionic catalyst in aqueous medium with an yield of 90%.
![]()
Question 11.
Explain the effects of temperature and pressure on the following equilibrium.
2NO2(g) ⇌ N2O4(g); ΔH = -57.2 kJmol-1
Answer:
At low temperature, the forward reaction of formation of N2O4 is preferred, as the reaction is exothermic and thus intensity of brown colour due to NO2 decreases. High temperature favours the reverse reaction of formation of NO2 and thus, the brown colour intensifies.
High pressure favours forward reaction as it is accompanied by decrease in number of moles and low pressure favours the backward reaction as it is accompanied by increase in number of moles.
Question 12.
Draw the structure of orthoboric acid. Why it is not a protonic acid?
Answer:
Boric acid has a layer structure in which ploanarBO3 units are joined by hydrogen bonds as shown below:
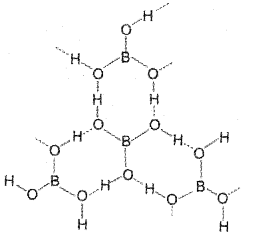
Boric acid is not a protic acid because it cannot ionise to give H+ ions. It acts as a Lewis acid by accepting electrons from a hydroxyl ion.
B(OH)3 + 2HOH → [B(OH)4]– + 2OH–
Question 13.
Find the molecular formula of the compound with molar mass. 78g mol-1 and empirical formula, CH.
Answer:
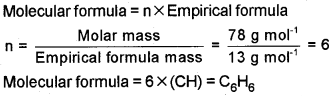
Question 14.
What is meant by entropy of a system? What happens to the entropy during the following changes?
a) A gas condenses into liquid.
![]()
Answer:
Entropy of a system is a measure of the degree of randomness or disorder of the system,
a) Entropy decreases
b) Entropy increases
Question 15.
Represent graphically, the variation of probability density (Ψ2(r)) as a function of distance (r) of the electron from the nucleus for 1s and 2s orbitals.
Answer:
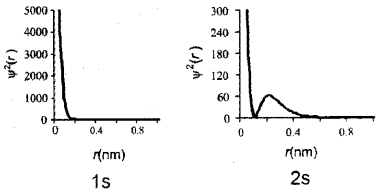
Question 16.
Derive an equation relating molar mass of an ideal gas with its density.
Answer:
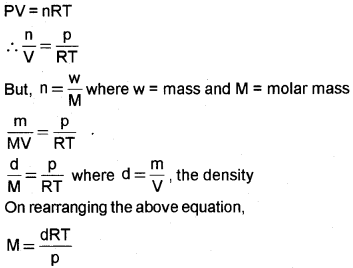
Question 17.
What is Wurtz reaction? Give an example.
Answer:
Methyl Alkyl halides on treatment with sodium metal in dry ethereal (free from moisture) solution give higher alkanes. This reaction is known as Wurtz reaction and is used for the preparation of higher alkanes containing even number of carbon atoms.
![]()
Question 18.
Define buffer solutions and write one example for an acidic buffer.
Answer:
The solutions which resist change in pH on dilution or with the addition of small amounts of acid or alkali are called buffer solutions.
A mixture of acetic acid and sodium acetate acts as an acid buffer around a pH 4.75.
Question 19.
Cycloheptatrienyl Cation is given below:
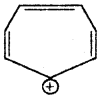
Is this ion aromatic or not? Justify the answer.
Answer:
Yes, cyclopentatrienyl cation is aromatic due to the following characteristics:
- Planarity
- Complete delocalisation of the π electrons in the ring
- Presence of (4n + 2)π = (4 × 1 + 2)π = 6π electrons in the ring.
Question 20.
Write the thermochemical equation corresponding to the standard enthalpy of formation of benzene. (Hint: ΔfH⊖ of benzene = + 49.0 kJmol-1)
Answer:
6C(graphite) + 3H2(g) → C6H6(l); ΔfH⊖ = +49.0 kJ mol-1
Answer any seven from question numbers 21 to 29. Each carries three scores. (Scores: 7 × 3 = 21)
Question 21.
Justify the following:
a) ‘Ne’has positive value for electron gain enthalpy.
b) The electron gain enthalpy of ‘F’ is lower than that of ‘Cl’.
c) The size of ‘Al3+’ is lower than that of ‘F’.
Answer:
a) ‘Ne’ is a noble gas. Noble gases have large positive electron gain enthalpies because the electron has to enter the next higher principal quantum level leading to a very unstable electronic configuration.
b) When an electron is added to F, the added electron goes to the smaller n = 2 quantum level and suffers significant repulsion from the other electrons present in this level. Whereas in the case of Cl, the electron is added to n = 3 quantum level in which it occupies a larger region of space and electron-electron repulsion is much less.
c) Al3+ and F– are isoelectronic. For isoelectronic species, the cation with greater positive charge will have a smaller radius because of the greater attraction of the electrons to the nucleus. The size of the ion decreases with increase in nuclear charge/atomic number. Al3+ has greater nuclear charge than F–.
Question 22.
Identify X, Y and Z in the following sequence of reactions:
![]()
Answer:
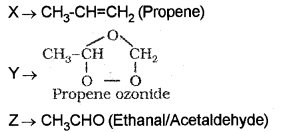
Question 23.
Calculate the mass of oxalic acid dihydrate (H2C2O4.2H2O) required to prepare 0.1 M, 250 ml of its aqueous solution.
Answer:
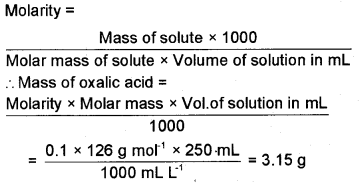
Question 24.
Balance the following Redox reaction by ion-electron method or oxidation number method (Acid medium)
![]()
Answer:
Step 1: The skeletal ionic equation is
Cr2O72-(aq) + SO32- → Cr3+(aq) + SO42-(aq)
Step 2: Assign oxidation numbers for Cr and S.
![]()
Step 3: Calculate the increase and decrease oxidation number and make them equal.
![]()
Step 4: As the reaction occurs in acidic medium add 8H+ on the left to make ionic charges equal.
Cr2O72-(aq) + 3SO32- + 8H+(aq) → 2Cr3+(aq) + 3SO42-(aq)
Step 5: Add 4H2O on the right to achieve balanced redox reaction.
Cr2O72-(aq) + 3SO32- + 8H+(aq) → 2Cr3+(aq) + 3SO42-(aq) + 4H2O(l)
(Or, correct balancing by ion-electron method.)
Question 25.
Explain any one method of preparation and structure of diborane.
Answer:
Diborane is prepared by treating boron trifluoride with LiAlH4 in diethyl ether.
4BF3 + 3LiAlH4 → 2B2H6 + 3LiF + 3AlF3
OR
Diborane is conveniently prepared in the laboratory by the oxidation of sodium borohydride with iodine.
2NaBH4 + l2 → B2H6 + 2Nal + H2
OR
Diborane is produced on an industrial scale by the reaction of BF3 with sodium hydride.
![]()
In diborane the four terminal hydrogen atoms and the two boron atoms lie in one plane. Above and below this plane, there are two bridging hydrogen atoms. Each boron uses sp3 hybrid orbitals for bonding. The four terminal B-H bonds are regular two centre-two electron bonds while the two bridge B-H-B bonds are three centre-two electrons bonds also known as banana bonds.
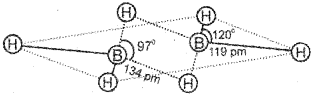
Question 26.
The value of equilibrium constant is useful to predict the extent of reaction and the direction of the reaction at a given stage. Explain.
Answer:
Predicting the extent of reaction: The numerical value of the equilibrium constant for a reaction indicates the extent of the reaction. A high value of K is suggestive of a high concentration of products and vice-versa.
If Kc > 103, products predominates over reactants, i.e., if Kc is very large, the reaction proceeds nearly to completion.
If Kc < 10-3, reactants predominate over products, i.e., if Kc is very small, the reaction proceeds rarely. If Kc is in the range of 10-3 to 103, appreciable concentrations of both reactants and products are present.
Predicting the direction of the reaction: The equilibrium contant helps in predicting the direction in which a given reaction will proceed at any state. For this we have to calculate the reaction quotient, Qc.
If Qc > Kc, the reaction will proceed in the direction of reactants (reverse reaction.)
If Qc < Kc, the reaction will proceed in the direction of the products (forward reaction).
If Qc = Kc, the reaction mixture is already at equilibrium.
Question 27.
Give a reason for the following:
a) H2O2 is stored in wax-lined glass or plastic vessels in dark.
b) Hard water is not suitable for laundry.
Answer:
a) H2O2 decomposes slowly on exposure to light.
2H2O2(l) → 2H2O(l) + O2(g)
In the presence of metal surfaces or traces of alkali present in glass containers, the above reaction is catalysed. It is, therefore, stored in wax-lined glass or plastic vessels in dark.
b) Hard water does not give lather with soap. It forms scum or precipitate with soap. Soap containing sodium stearate reacts with hard water to precipitate of Ca/Mg stearate. Hence, hard water is not suitable for laundry.
Question 28.
Calculate the total pressure in a mixture of 3.5 g of dinitrogen and 16g of dioxygen confined in a vessel of 2 dm3 at 27°C.
(R = 0.083 bar dm3 K-1 mol-1)
Answer:
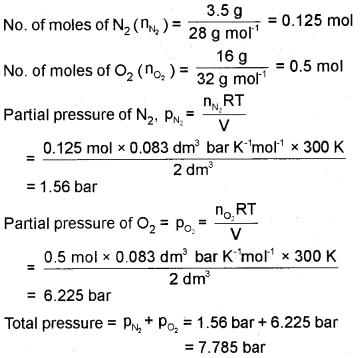
Question 29.
The reaction of cyanamide (NH2CN) with dioxygen was carried out in a bomb calorimeter and Au was found to be -742.7kJmol-1, at 298 K. Calculate enthalpy change for the reaction at 298 K.
![]()
Answer:
ΔH = ΔU + ΔngRT
Δng = 2 – 2.5 = -0.5
= -742.7 × 103 J mol-1 + (- 0.5 × 8.314 K-1mol-1 × 298 K)
= -743938 J mol-1 = -743.938 kJ mol-1
Answer any three from question numbers 30 to 33. Each carriesfourscores. (Scores: 3 × 4 = 12)
Question 30.
Write the molecular orbital electronic configurations of N2 and O2 and calculate their bond orders. Give a comparison of their stability and magnetic behaviour.
Answer:
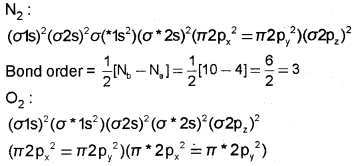
Bond order= 12[Nb – NB] = 12[10 – 6] = 42 = 2
Bond order is directly proportional to stability.
N2 with higher bond order is more stable than O2 with lower bond order.
N2 is diamagnetic since it has no unpaired electrons.
O2 is paramagnetic since it contains two unpaired electrons in π*2px and π*2py molecular orbitals.
Question 31.
Briefly describe the principles of the following techniques, taking an example in each case.
a) Crystallization
b) Simple distillation
c) Distillation under reduced pressure
d) Paper chromatography
Answer:
a) Crystallisation: This is one of the most commonly used techniques for the purification of solid organic compounds. It is based on the difference in the solubilities of the compound and the impurities in a suitable solvent. The impure compound is dissolved in a solvent in which it is sparingly soluble at room temperature but appreciably soluble at higher temperature. The solution is concentrated to get a nearly saturated solution. On cooling the solution, pure compound crystallises out and is removed by filtration, e.g. Ethanol can be used for purification of sugar containing impurities of common salt which does not dissolve in ethanol.
b) Simple distillation: This method is used to separate (i) volatile liquids from nonvolatile impurities and (ii) the liquids having sufficient difference in their boiling points. Liquids having different boiling points vaporise at different temperatures. The vapours are cooled and the liquids so formed are collected separately. The liquid mixture is taken in a round bottom flask and heated carefully. On boiling, the vapours of lower boiling component are formed first. The vapours are condensed by using a condenser and the liquid is collected in a receiver. The vapours of higher boiling component form later and the liquid can be collected separately, e.g. Chloroform (b.p 334 K) and aniline (b.p. 457 K) are easily separated by the technique of distillation.
c) Distillation under reduced pressure: This method is used to purify liquids having very high boiling points and those, which decompose at or below their boiling points. Such liquids are made to boil at a temperature lower than their normal boiling points by reducing the pressure on their surface, e.g. Glycerol can be separated from spent-lye in soap industry by using this technique.
d) Paper chromatography: It is a type of partition chromatography. In this, a special quality paper known as chromatography paper is used. It contains water trapped in it, which acts as the stationary phase. A strip of chromatography paper spotted at the base with the solution of the mixture is suspended in the suitable solvent or a mixture of solvents.
This solvent acts as the mobile phase. The solvent rises up the paper by capillary action and flows over the spot. The paper selectively retains different components according to their differing partition in the two phases. The paper strip so developed is known as a chromatogram. The spots of the separated coloured compounds are visible at different heights from the position of initial spot on the chromatogram. The spots of the separated colourless compounds may be observed either under ultraviolet light or by the use of an appropriate spray reagent.
e.g. separation of the components present in small amounts from a liquid mixture.
Question 32.
Give the postulates of Bohr model of hydrogen atom. Also write two merits and two limitations of this model.
Answer:
Bohr’s model for hydrogen atom is based on the following postulates:
i) The electron in the hydrogen atom can move around the nucleus in a circular path of fixed radius and energy. These paths are called orbits, stationary states or allowed energy states and are arranged concentrically around the nucleus.
ii) The energy of an electron in the orbit does not change with time. However, the electron will move from a lower stationary state to a higher stationary state when required amount of energy is absorbed by the electron or energy is emitted when electron moves from higher stationary state to lower stationary state. The energy change does not take place in a continuous manner.
iii) The frequency of radiation absorbed or emitted when transition occurs between two stationary states that differ in energy by ΔE, is given by:
v=ΔEh=E2−E12
Where E1 and E2 are the energies of the lower and higher allowed energy states respectively. This expression is known as Bohr’s frequency rule.
iv) The angular momentum of an electron in a given stationary state can be expressed as in equation,
mevr= nh2π, n = 1,2, 3
Thus an electron can move only in those orbits for which its angular momentum is integral
multiple of h/2π that is why only certain fixed orbits are allowed.
Merits:
- Bohr’s model could explain the stability of an atom.
- Bohr’s model helped in calculating energy of an electron in a particular orbit of hydrogen.
- Bohr’s model could explain the atomic spectrum of hydrogen.
Limitations:
- It fails to account for the finer details of the hydrogen atom spectrum observed by using sophisticated spectroscopic techniques.
- This model is unable to explain the spectrum of atoms other than hydrogen.
- Bohr’s theory was also unable to explain the splitting of spectral lines in the presence of magnetic field (Zeeman effect) or an electric field (Stark effect).
- It could not explain the ability of atoms to form molecules by chemical bonds.
(Any two limitations.)
Question 33.
Account for the following:
a) Blue coloured solutions are obtained when alkali metals are dissolved in liquid ammonia.
b) ‘Li’ and ‘Mg’ show similar properties.
c) Aqueous solution of Na2CO3 is alkaline.
d) BeSO4 and MgSO4 are readily soluble in water.
Answer:
a) The alkali metals dissolve in liquid ammonia giving deep blue solutions which are conducting in nature.
M + (x + y)NH3 → [M(NH3)x]+ + [e(NH3)y]–
The blue colour of the solution is due to the ammoniated electron which absorbs energy in the visible region of light and thus imparts blue colour to the solution.
b) The similarity between lithium and magnesium is because of their similar sizes: atomic radii, Li = 152 pm, Mg = 160 pm; ionic radii: Li+ = 76 pm, Mg2+ = 72 pm. Both Li and Mg have similar electronegativities and they show diagonal relationship.
c) It is due to the formation of NaOH on hydrolysis. Na2CO3 is a salt of weak acid (H2CO3) and a strong base (NaOH). Carbonate part of sodium carbonate gets hydrolysed by water to form an alkaline solution.
CO32- + H2O → HCO3– + OH–
d) This is because hydration enthalpy (ΔhydH) is greater than lattice enthalpy (ΔlatticeH). BeSO4 and MgSO4 are readily soluble in water; the solubility decreases from CaSO4 to BaSO4. The greater hydration enthalpies of Be2+ and Mg2+ ions overcome the lattice enthalpy factor and therefore their sulphates are soluble in water.
