Kerala Plus One Chemistry Previous Year Question Paper March 2019 with Answers
| Board | SCERT |
| Class | Plus One |
| Subject | Chemistry |
| Category | Plus One Previous Year Question Papers |
Time Allowed: 2 hours
Cool off time: 15 Minutes
Maximum Marks: 60
General Instructions to Candidates:
- There is a ‘cool off time’ of 15 minutes in addition to the writing time.
- Use the ‘cool off time’ to get familiar with questions and to plan your answers.
- Read the instructions carefully.
- Read questions carefully before answering.
- Calculations, figures and graphs should be shown in the answer sheet itself.
- Malayalam version of the questions is also provided.
- Give equations wherever necessary.
- Electronic devices except non programmable calculators are not allowed in the Examination Hall.
Answer all questions from 1 to 7. Each question carry one score. (7 × 1 = 7)
Question 1.
The lowest hypothetical temperature at which gases are supposed to occupy zero volume is called …………
Answer:
-273.15°c. Absolute zero temperature.
Question 2.
Which among the following is a molecular hydride?
a) LiH
b) NH3
c) CrH
d) LaH2.87
Answer:
b) NH3
Question 3.
Give the IUPAC name of ………
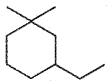
Answer:
3, Ethyl 1, 1 – dimethyl cyclo hexane
Question 4.
Predict the product obtained by the reaction of Li with O2.
Answer:
Li2O
Question 5.
According to the first law of thermodynamics, for an isolated system, Δu = ……….
Answer:
Zero
Question 6.
The minimum value for the product of uncertainties in position and momentum of a moving microscopic particle is equal to …………
Answer:
\(\frac{\mathrm{h}}{4 \pi}\)
Question 7.
Round off 0.0525 to a number with two significant figures.
Answer:
0.052
Answer any ten questions from 8 to 20. Each carries twoscores (10 × 2 = 20)
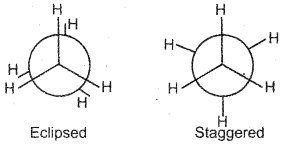
Question 8.
Draw the Newman projections of the eclipsed and staggered conformations of ethane molecule.
Answer:
Newman Projection Formula:
Question 9.
Calculate the pH of 1 × 10-2 molar aqueous solution of H2SO4
Answer:
PH = -log[H+]
-log[2 × 10-2] = 1.699
Question 10.
Among NaCl, BeCl2 and AlCl3 which one is more covalent? Justify the answer.
Answer:
AlCl3. Fajans Rule says that smaller the size and greater the charge of cation greater will be the polarising power, hence the covalent character.
Question 11.
Differentiate homolytic cleavage from heterolytic cleavage of covalent bonds.
Answer:
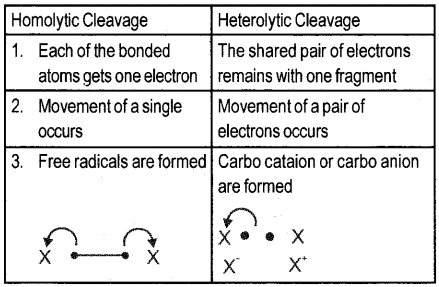
Question 12.
Mention two observations which could not be explained by the wave nature of electromagnetic radiations.
Answer:
Black body radiation, photo electric effect, atomic spectra, hot body radiation, line spectrum, reflection, Compton effect, (any two).
Question 13.
‘Chlorine has the most negative electron gain enthalpy’. Justify the statement.
Answer:
In chlorine the incoming electron enters into 3p orbital which is larger than 2p of Fluorine atom. So repulsion is low and Cl has highest electron gain enthalpy.
Question 14.
Examine the chemical equilibrium,
![]()
Write the expression for equilibrium constant (Kc) for the above equilibrium. What happens to Kc, if the balanced equation is multiplied throughout by a factor, 2.
Answer:
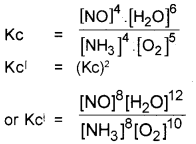
Question 15.
Give the chemical equations for the steps involved In the ozonolysis of propene.
Answer:
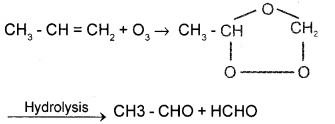
Question 16.
Draw the structure of Diborane. Write a note on the nature of bonds present in it.
Answer:
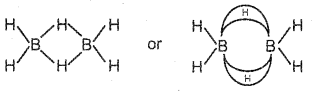
Two types of bonds are present in diborane.
i) Two centre – two electron bonds (2c – 2e)
ii) Three centre – two electron bonds (3c – 2e)
In B2H6 four hydrogen atoms in one plane. These hydrogen atoms are known as terminal hydrogen. The other two hydrogen atoms which is above and below the plane are called bridging H atoms.
Question 17.
What is meant by spontaneous processes? Give the criterion of spontaneity in terms of ΔG for a process taking place at constant temperature and pressure.
Answer:
A process that takes place without the help of any external agency is called a spontaneous process. The conditions for a spontaneous process is
ΔG = -ve or ΔG < 0
Question 18.
Give the relation between molar-mass of a gas (m) and its density (d). How are the densities of O2(g). and CH4(g) related, if they are kept at the same temperature and pressure?
Answer:
PM = dRT
P – Pressure, M – Molar Mass, d – density, R – universal gas constant, T – Temparature
d1 = 2d2
\(\frac{M_{1}}{M_{2}}=\frac{d_{1}}{d_{2}} \Rightarrow \frac{32}{16}=\frac{d_{1}}{d_{2}}\)
Density = 2 : 1
Question 19.
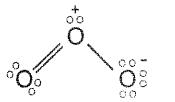
Represent, the Lewis structure of O3 molecule and assign the formal charge on each atom.
Answer:
Lewis structure of ozone is
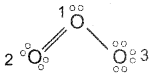
Formal Charge = Total no. of valence electrons on the free atom – Total no. of lone pair of electrons – \(\frac{1}{2}\) (Total no. of bonding electrons)
Formal charge on first O atom = 6 – 2 – \(\frac{1}{2}\)(6) = 1
Formal charge on 2nd O atom = 6 – 4 – \(\frac{1}{2}\)(4) = 0
Formal charge on 3rd O atom = 6 – 6 – \(\frac{1}{2}\)(2) = 1
Question 20.
Identify the positions of Al (z = 13) and S (z = 16) in the periodic table with the help of their electronic configurations. Predict the formula of the compound formed between them.
Answer:
Electronic configuration of Al13 is 1s2, 2s2, 2p6, 3s2, 3p1. It is a p block element because last electron enters in p subshell.
Period no. 3 (Highest principal quantum no.)
Group No. : 13 (No. of valence electrons + 10)
Electronic cinfiguration of S16 – 1s2, 2s2, 2p6, 3s2, 3p4.
p block element, period No. 3 and group no. 16
The compound formed by Al and S isAl2S3 because valency of Al = 3 and that of S = 2.
Answer any seven questions from 21 to 29. Each carries three scores. (7 × 3 = 21)
Question 21.
Give reasons for the anomalous behaviour of Li. Write any four points of similarities between Li and Mg.
Answer:
Anomalous properties of Li are due to its small size, high ionisation enthalpy, high polarising power and absence of vacant d orbitals.
Anomalous properties of Li and Magnesium
- Both Li and Mg are harder
- Both react slowly with water
- They do not form super oxides
- Their chlorides are deliquescent
- Their bicarbonates are stable only in solution
Question 22.
Explain the hydrolysis of different types of salts with the help of examples and comment on the pH of the resulting solutions in each case.
Answer:
There are four types of salts
i) Hydrolysis of salts of strong base and weak acid,
eg. CH3COONa, Na2CO3, KCN
These salts on hydrolysis give strong base and weak acid, hence the pH of resulting solution is as that of base, greater than 7.
ii) Hydrolysis of salts of weak base and week acid, eg. NH4Cl, NH4NO3, CUSO4 etc.
These salts on hydrolysis gives weak base and strong acid and there fore pH of resulting solution is of less than 7.
iii) Hydrolysis of salts of weak base and strong acid, eg. Ammonium Acetate (CH3COONH4), ammonium carbonate (NH4)2CO3. These salts on hydrolysis give neutral solution.
iv) Hydrolysis of salt of strong acid and strong base. These salts do not undergo hydrolysis and the solution is neutral.
Question 23.
a) What is meant by acid rain?
b) Explain the chemistry behind the formation of acid rain.
c) What are the harmful effects of acid rain?
Answer:
a) If the pH of the rainwater drops is below 5.6 it is called acid rain.
b) Oxides of nitrogen and sulphur are mainly responsible for acid rain.
S + O2 → SO2
5O2 + O2 → SO3
5O3 + H2O → H2SO4
or
N2 + O2 → 2NO
NO + O2 → NO2
NO2 + H2O → HNO3
c) i) Acid rain is harmful for agriculture, trees and plants.
ii) It causes skin cancer
iii) It affects plants and animal life.
iv) Damages buildings and other structures
Question 24.
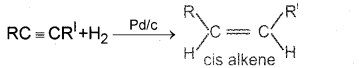
Alkynes can be converted selectively into cis-alkenes and trans-alkenes. Explain with suitable examples.
Answer:
Lindlar catalyst gives cis product.
If the reduction is carried out using sodium in liquid ammonia, we get trans alkene.
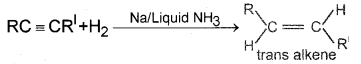
Question 25.
A reaction mixture for the production of NH3 gas contains 250g of N2 gas and 50g of H2 gas under suitable conditions. Identify the limiting reactant, if any and calculate the mass of NH3 gas produced.
Answer:
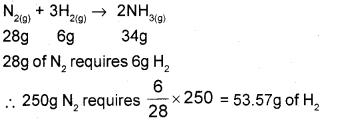
But we have only 50g of H2 gas.
6g H2 requires 28g N2.
50g H2 requires \(\frac{28}{6}\) × 50 = 233.33g N2
H2 is completely consumed, so it is the limiting reagent.
Amount of ammonia formed = 50 + 233.33 = 283.33g
Question 26.
Write the molecular orbital electronic configuration, of N2 and O2 molecules. Compare the stability and magnetic behaviour of these molecules on the basis of M.O theory.
Answer:
Molecular orbital configuration of N2 is
σ1s2σ*1s2σ2S2σ*2s2π2px2 = π2py2σ2pz2
Bond order = \(\frac{1}{2}\)(nb – na)
= \(\frac{1}{2}\)(10 – 4) = 3
Due to the presence of paired electron, the molecule is diamagnetic.
Molecular orbital configuration of O2
σ1s2σ*1s2σ2s2σ*2s2σ2pz2π2px2
= π2py2π*2px1 = π*2py1
Bond order = \(\frac{1}{2}\)(nb – na)
= \(\frac{1}{2}\)(10 – 6) = 2
Due to the presence of unpaired electron, the O2 molecule is paramagnetic.
As the bond order increases stability increases. So N2 is more stable than O2.
Question 27.
a) Why do real gases deviate from ideal behaviour?
b) Write the conditions under which gases deviate from ideality.
c) Define Boyle point.
Answer:
a) Due to two wrong assumptions
- There is no force of attraction between the gas molecules.
- The actual volume of gas molecule is negligible compared to the total volume of the gas.
b) High pressure and low temperature.
c) It is the temperature at which the real gases obey ideal gas equation over a range of pressure.
Question 28.
Give the structure, a method of preparation and a chemical reaction of H2O2
Answer:
Open book like structure or laptop structure.
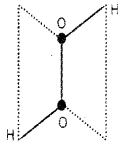
Chemical reaction
H2O2 decomposes slowly on exposure to light.
2H2O2(l) → 2H2O(l) + O2(g)
Preparation of hydrogen peroxide
By acidifying barium peroxide with H2SO4
BaO2.8H2O(s) + H2SO4(aq) → BaSO4 + H2O2(aq) + 8H2O(I)
Question 29.
Balance the following Redox process by ion-electron method or oxidation number method.
Answer:
Oxidation no. Method
i) Write the equation P4 + OH– → PH3 + HPO–2
ii) Write the oxidation no. of each element and identify atoms which undergo oxidation or reduction.
P04 + OH- → P-3H3 + H-2PO2
iii) Calculate the change in oxidation no. per atom and equate them by multiplying with suitable coefficient.
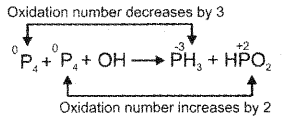
2P4 + 3P4 + OH– → PH3 + HPO–2
iv) Balance all the atoms except oxygen and hydrogen
2P4 + 3P4 + OH– → 8PH3 + 12HPO–2
v) Now equate the ionic charges on both sides. Since the reaction occurs in basic medium and 11OH– ions on the reactant side.
2P4 + 3P4 + OH– + 11OH– → 8PH3 + 12HPO–2
vi) Now balance the hydrogen atoms by adding 12 water (H2O) molecules on L.H.S.
5P4 + 12OH– + 12H2O → 8PH3 + 12HPO–2
Now, the equation is balanced.
OR
Ion Electron Method:
The skeletal equation is P4 + OH– → PH3 + HPO–2 Presence of OH– in the equation indicates the reaction is taking place in basic medium.
i) Write the oxidation no. of each element and find out the substance oxidised and reduced.
PO4 + OH– → P-3H3 + H+2PO2
P4 undergoes oxidation and reduction at the same time. This is known as disproportionation.
ii) Separate the equation into oxidation half reaction and reduction half reaction.
Oxidation half: P4 → HPO–2
Reduction half: P4 → PH3
iii) Balance the atoms other than O and H in each half reaction individually.
Oxidation half: P4 → 4HPO–2
Reduction half: P4 → 4PH3
iv) Now balance O and H atoms. Add H2O to balance O atoms and H+ to balance H atoms.
Since the reaction occurs in basic medium add equal no. of OH– ions on both sides of the equation.
Oxidation half:
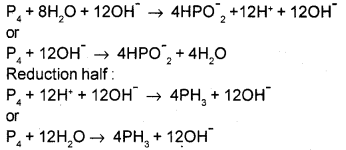
v) Now balance the ionic charges. For this add electrons to one side of the half reaction.
Oxidation half:
P4 + 12OH– → 4HPO–2 + 4H2O + 8e–
Reduction half:
P4 + 12H2O + 12e– → 4PH3 + 12OH–
vi) Now add the two half reactions after equating the electrons.
Oxidation half:
(P4 + 12OH– → 4HPO–2 + 4H2O + 8e–) × 3
Reduction half:
(P4 + 12H2O + 12e– → 4PH3 + 12OH–) × 2
Overall reaction is
5P4 + 12OH– + 12H2O → 12HPO–2 + 8PH3
Answer any three questions from 30 to 33. Each carries four scores. (3 × 4 = 12)
Question 30.
Explain quantum numbers. Give the importance of quantum numbers in Pauli’s Exclusion Principle.
Answer:
Quantum numbers are the address of the electron. It is used to explain the size, shape, spin and orientation of an electron.
Quantum numbers are four
i) Principal quantum no (n) gives us idea about size and energy of electron in an orbit. The values can be 1, 2, 3, 4, 5, etc.
ii) Azimuthal Quantum No. (l): It gives the shape of the orbital. It is also called the orbital angular momentum quantum no. I can have values from 0 to (n – 1).
iii) Magnetic quantum No. (m): It gives orientation of orbitals in space, m can have values from -l to +l including zero. There are (2l + 1) possible values.
iv) Spin Quantum no. (s): It gives the spin orientation of electrons. It can have two values +\(\frac{1}{2}\) or –\(\frac{1}{2}\)
Paul’s exclusion principle: No two electrons in an atom can have same set of four quantum nos.
Or
If two electrons have same values for n, I and m they should have different values for s. ie. s = +\(\frac{1}{2}\) for first electron and –\(\frac{1}{2}\) for second electron.
Question 31.
a) What are silicones?
b) Write the chemical equations showing the steps involved in the manufacture of silicones.
c) How can the chain length of silicones be controlled during their synthesis?
Answer:
a) Silicones are organo silicon polymers with -R2SiO- repeating unit.
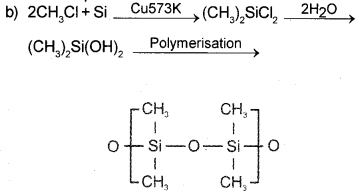
c) By adding (CH3)3SiCl the chain length can be controlled.
Question 32.
Briefly explain the different types of structural isomerism shown by organic compounds with suitable examples.
Answer:
Structural isomerism are of four types
a) Chain Isomerism: Isomers differ in carbon chain
eg. Pentane -C6H12
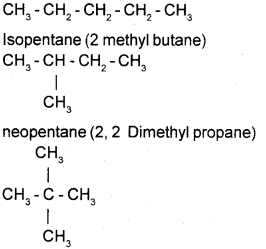
b) Position Isomerism: Isomers differ in the position of the side chain or functional group.
eg. 1 butanol – C4H10O
CH3-CH2-CH2-CH2-OH
2 Butanol
CH3-CHOH-CH2-CH3
c) Functional group Isomerism: Isomers which differ in the functional group.

d) Metamerism: Isomers which differ in the number of carbon atoms around the functional group.
eg. C5H12O
Methoxy Propane CH3 – O – CH2 – CH2 – CH3
Ethoxy Ethane CH3 – CH2 – O – CH2 – CH3
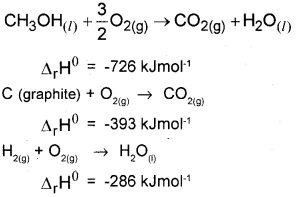
Question 33.
a) State Hess’ law of constant heat summation.
b) Calculate the standard enthalpy of formation of CH3OH(l) from the following data: