Kerala Plus One Chemistry Notes Chapter 8 Redox Reactions
Introduction
The reaction which involve both oxidation and reduction reactions is called Redox reaction.
Classical Idea Of Redox Reactions Oxidation And Reduction Reactions
“Oxidation” is defined as the addition of oxygen/electronegative element to a substance or removal of hydrogen/electropositive element from a substance. Examples of oxidation:
- Addition of oxygen 2Mg + O2 → 2MgO
- Removal of hydrogen 2H2S + O2 → 2S + 2H2O
- Addition of electronegative element Mg + Cl2 → MgCl2
The term reduction been broadened these days to include removal of oxygen/electronegative element from a substance or addition of hydrogen /electropositive element.
- Removal of electronegative element FeCl3 + H2 → 2FeCl2 + 2HCl
- Removal of Oxygen (2H2O → 2Hg + O2)
- Addition of Hydrogen (H2 + Cl2 → 2HCl)
Redox Reactions In Terms Of Electron Transfer Reactions
According to electronic concept, the processes which involves loss of electrons are called oxidation reactions. Similarly, processes which involve gain of electrons are called reduction reactions.
The atom which reduced, act as oxidising agent and the atom which oxidised act as reducing agent.For example;
2Na(s) + Cl2(g) → 2Na+Cl–(s) or 2NaCl(s)
Here Na is oxidised and Cl is redused.
Competitive Electron Transfer Reaction
Place a strip of metallic zinc in an aqueous solution of copper nitrate. You may notice that the strip becomes coated with reddish metallic copper and the blue colour of the solution disappears. Formation of Zn2+ ions among the products can easily be judged when the blue colour of the solution due to Cu2+ has disappeared. The reaction is,
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) zinc is oxidised, releasing electrons, something must be reduced, accepting the electrons lost by zinc. Copper ion is reduced by gaining electrons from the zinc.
Oxidation Number
Oxidation number of an element may be defined as the charge which an atom of the element has or appears so have when present in the combined state in a compound.
- Electrons shared between two like atoms are divided equally between the sharing atoms.
- Electrons shared between two unlike atoms are counted with the more electronegative atom. Atoms can assume positive, zero or negative values of oxidation numbers depending on their state of combination. Oxidation number can be a fraction in some cases.
The rules for calculation of oxidation number are:
1. In elements, in the free or the uncombined state, each atom bears an oxidation number of zero. Evidently each atom in H2 has the oxidation number zero.
2. For ions composed of only one atom, the oxidation number is equal to the charge on the ion. Thus Na+ ion has an oxidation number of +1, Mg2+ion, +2, Fe3+ ion, +3, Cl– ion, -1, O2- ion, -2; and so on. In their compounds all alkali metals have oxidation number of +1, and all alkaline earth metals have an oxidation number of +2. Aluminium is regarded to have an oxidation number of +3 in all its compounds.
3. The oxidation number of oxygen in most compounds is-2. However, we come across two kinds of exceptions here.in peroxides (e.g., H2O2, Na2O2), each oxygen atom is assigned an oxidation number of—1, in superoxides (e.g., KO2, RbO2) each oxygen atom is assigned an oxidation number of -(½). The second exception appears rarely, i.e. when oxygen is bonded to fluorine. In such compounds e.g., oxygen difluoride (OF2) and dioxygen difluoride (O2F2), the oxygen is assigned an oxidation number of +2 and +1, respectively. The number assigned to oxygen will depend upon the bonding state of oxygen but this number would now be a positive figure only.
4. The oxidation number of hydrogen is +1, except when it is bonded to metals in binary compounds (that is compounds containing two elements). For example, in LiH, NaH, and CaH2, its oxidation number is —1.
5. In all its compounds, fluorine has an oxidation number of-1. Other halogens (Cl, Br, and I) also have an oxidation number of-1, when they occur as halide ions in their compounds. Chlorine, bromine and iodine when combined with oxygen, for example in oxoacids and oxoanions, have positive oxidation numbers.
6. The algebraic sum of the oxidation number of all the atoms in a compound must be zero. In polyatomic ion, the algebraic sum of all the oxidation numbers of atoms of the ion must equal the charge on the ion. Thus, the sum of oxidation number of three oxygen atoms and one carbon atom in the carbonate ion, (CO3)2- must equal -2. A term that is often used interchangeably with the oxidation number is the oxidation state. Oxidation state of a metal is a compound is sometimes represented by Stock notation. According to this, the oxidation number is written as Roman numeral in parenthesis after the symbol of the metal in the molecular formula. e.g.,Fe(ll)0, Sn(IV), Cl4,Mn(IV)O2.
Problem
Using Stock notation, represent the following compounds HAUCl4, Ti2O, FeO, Fe2O3, Cul, CuO, MnO and MnO2.
Solution
By applying various rules of calculating the oxidation number of the desired element in a compound, the oxidation number of each metallic element in its compound is as follows:
HAuCl4 → Au has 3
Tl2O → Tl has 1
FeO → Fe has 2
Fe2O3 → Fe has 3
Cul → Cu has 1
CuO → Cu has 2
MnO → Mn has 2
MnO2 → Mn has 4
Therefore, these compounds may be represented as
HAU(III)Cl4, Tl2(I)O, Fe(II)O, Fe2(III)O3, Cu(I)l, Cu(II)O, Mn(II)O, Mn(IV)O2.
In terms of oxidation number, oxidation may be defined as a chemical change in which there occurs an increase in the oxidation number of an atom or atoms. Reduction may be defined as a chemical change in which there occurs a decrease in the oxidation number of an atom or atoms. Thus, a redox reaction may be defined as a reaction in which the oxidation number of atoms undergoes a change.
Types Of Redox Reactions
1. Combination Reactions:
A combination reaction may be denoted in the manner
A + B → C
![]()
2. Decomposition Reaction:
Decomposition reactions are the opposite of combination reactions.
For example, 2H2O → 2H2 + O2
3. Displacement Reaction:
In a displacement reaction, an ion (or an atom) in a compound is replaced by an ion (or an atom) of another element. It may be denoted as:
X +YZ → XZ + Y
Displacement reactions fit into two categories:
metal displacement and non-metal displacement.
a) Metal displacement:
A metal in a compound can be displaced by another metal in the uncombined state.
CuSO4(aq) + Zn(s) → Cu(s) + ZnSO4(aq)
b) Non-metal displacement:
The non-metal displacement redox reactions include hydrogen displacement and a rarely occurring reaction involving oxygen displacement.
2Na(s) + 2H2O(I) → 2NaOH(aq) + H2(g)
The power of these elements as oxidising agents decreases as we move down from fluorine to iodine in group 17 of the periodic table.
Note:
fluorine is the strongest oxidising agent; there is no way to convert F– ions to F2 by chemical means. The only way to achieve F2 from F– is to oxidise electrolytically,
4. Disproportionation Reactions:
In a disproportionation reaction an element in one oxidation state is simultaneously oxidised and reduced.
Balancing Of Redox Reactions
There are two ways to balance a redox equation.
They are oxidation number method and Half Reaction Method.
a) Oxidation Number Method
The various steps involved in this method are:
- Write the skeletal equation and assign oxidation numbers to each element. Identify the elements undergoing change in oxidation number.
- Find out the increase or decrease of oxidation number per atom. Multiply the increase or decrease of oxidation number with number of atoms undergoing the change.
- Multiply the formulae of the oxidising agent and the reducing agent by suitable integers so as to equalize the total increase or decrease in oxidation number as determined in the above step.
- Balance the equation with respect to all atoms other the term reduction has than oxygen and hydrogen.
- Balance oxygen atoms by adding equal number of H2O molecules to the side deficient in oxygen atoms.
- For reaction taking place in acidic medium, add H+ ions to the side of deficient in hydrogen atoms.
- For reaction taking place in basic medium, add H2O molecules to the side deficient in hydrogen atoms and simultaneously add equal number of OH ions on the other side of the equation.
Problem
Permanganate ion reacts with bromide ion in basic medium to give manganese dioxide and bromate ion. Write the balanced ionic equation forthe reaction.
Solution:
The skeletal ionic equation is:
MnO4–(aq) + Br–(aq) → MnO2(s) + BrO3–(aq)
Assign oxidation numbers for Mn and Br
![]()
this indicates that permanganate ion is the oxidant and bromide ion is the reductant.
Calculate the increase and decrease of oxidation number, and make the increase equal to the decrease.
![]()
As the reaction occurs in the basic medium, and the ionic charges are not equal on both sides, add 2 OH– ions on the right to make ionic charges equal.
2MnO4–(aq) + Br–(aq) → 2MnO2(s) + BrO3–(aq) + 2OH–(aq)
Finally, count the hydrogen atoms and add appropri- ‘ ate number of water molecules (i.e. one H20 molecule) on the left side to achieve balanced redox change.
2MnO4–(aq) + Br–(aq) → 2MnO2(s) + Br03–(aq) + 2OH–(aq)
b) Half Reaction Method
This method involves identifying the oxidation and reduction reactions in the given skeletal equation and then splitting the reaction accordingly as two half reactions. Each half reaction is then balanced systematically in various steps as outlined below.
Step 1.
Write the skeletal equation and identify the oxidant and reductant.
Step 2.
Write the half reactions for oxidation and reduction separately.
Step 3.
Balance the half reaction with respect to atoms that undergo change in oxidation number. Add electron to whichever side is necessary, to make up for difference in ON.
Step 4.
Balance O-atoms by adding proper number of H2O molecules to the side deficient in oxygen atoms.
Step 5.
For ionic equations in acid medium, add sufficient H+ ions to the side deficient in hydrogen. If the reaction occurs in basic medium, add sufficient H2O molecules to the side deficient in H atoms to balance H atoms and equal number of hydroxyl ions on the opposite side.
Step 6.
Equalise the number of electrons lost or gained by multiplying the half reaction with suitable integer and add the half reactions to get the final balanced equation.
Problem
Permanganate (VII) ion, MnO4– in basic solution oxidises iodide ion, l– to produce molecular iodine (l2) and manganese (IV) oxide (MnO2). Write a balanced ionic equation to represent this redox reaction.
Solution:

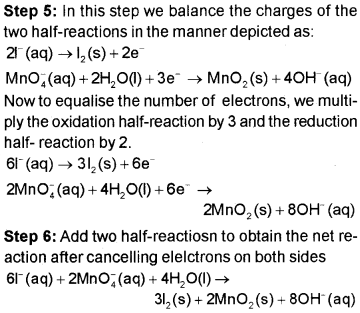
Redox Reactions As The Basis For Titrations
In redox systems, the titration method can be adopted to determine the strength of a reductant/ oxidant using a redox sensitive indicator. The usage of indicators in redox titration is illustrated below:
1. In one situation, the reagent itself is intensely coloured, e.g., permanganate ion, MnO4–. Here MnO4– – acts as the self indicator. The visible endpoint, in this case, is achieved after the last of the reductant (Fe2+ or C2O42-) is oxidised and the first lasting tinge of pink colour appears at MnO4– concentration as low as 10-6 mol dm-3 (10-6 mol L-1), This ensures a minimal ‘overshoot’ in colour beyond the equivalence point, the point where the reductant and the oxidant are equal in terms of their mole stoichiometry.
2. If there is no dramatic auto-colour change (as with Mn04 – titration), there are indicators which are oxidised immediately after the last bit of the reactant is consumed, producing a dramatic colour change. The best example is afforded by Cr2072-, which is not a self-indicator, but oxidises the indicator substance diphenylamine just after the equivalence point to produce an intense blue colour, thus signalling the endpoint.
Redox Reactions And Electrode Pro-Cesses
When zinc rod is dipped in copper sulphate solution, zinc gets oxidised to Zn2+ while Cu2+ ions are reduced to Cu due to direct transfer of electrons. However, if a zinc rod dipped in ZnSO4 solution taken in a breaker is connected externally by a conducting wire to a copper rod placed in CuSO4 solution in another beaker, electrons are transferred indirectly from Zn to Cu. Now, each beaker contains both the oxidised and reduced form of the same substance ‘ called a redox coupe. In this experiment the redox couples developed are Zn2+/Zn and Cu2+/Cu When the solutions in the two beakers (called electrodes) are joined by a salt bridge (a U-tube containing a solution of KCl, solidified in presence of agar-agar), electrons flow from Zn to Cu while current flows in the reverse direction. The salt bridge provides electrical continuity between the solutions without allowing them to mix with each other. The flow of current is due to a potential difference between Cu and Zn electrodes (or half cells). This experimental set up gives an electrochemical cell or galvanic cell.
The potential of an electrode is a measure of its ability to lose (oxidation) or gain (reduction) electrons. When the concentrations of solutions in the half cells are unity and the temperature is 298 K, the potential of each electrode is known as Standard Electrode Potential (E°). By convention, E° of hydrogen electrode is zero volts and the potential of other electrodes will be a measure of the relative tendency of the active species to be in oxidised/reduced form. A negative E°shows that the redox couple is a stronger reducing agent than H+/H2 couple.
A positive E° shows that the redox couple is a weaker reducing agent than H+/H2 couples. The values of standard reduction potentials of various electrodes are given in the increasing order in an electrochemical series (electromotive series)
