Kerala Plus One Chemistry Notes Chapter 4 Chemical Bonding and Molecular Structure
Introduction
Matter is made up of different type of elements. The attractive force which holds the constituents together is called a chemical bond.
Kossel-Lewis Approach To Chemical Bonding
The bond between constituents are formed by the sharing of a pair of electrons or their transfer. G.N. Lewis introduced simple notations to represent these outer shell electrons in an atom. These notations are called Lewis symbols.
![]()
Significance of Lewis Symbols :
The number of dots around the symbol represents the number of valence electrons. This number of valence electrons helps to calculate the common or group valence of the element. The group valence of the elements is generally either equal to the number of dots in Lewis symbols or8 minus the number of dots or valence electrons.
Kossel, in relation to chemical bonding, drew attention to the following facts:
The bond formed, as a result of the electrostatic attraction between the positiveand negative ions was termed as the electrovalent bond. The electrovalence is thus equal to the number of unit charge (s) on the ion.
In terms of Lewis structures
![]()
1. According to electronic theory of chemical bond¬ing, atoms can combine either by transfer of valence electrons from one atom to another (gaining or losing) or by sharing of valence electrons in order to have an octet in their valence shells. This is known as octet rule.
2. Covalent Bond, Langmuir in 1919 refined the Lewis postulations by abandoning the idea of the stationary cubical arrangement of the octet, and by introducing the term covalent bond.
By Lewis – Langmuir theory the formation of chlorine molecule is as follows :
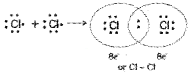
In water molecule covalent bond is as follows:
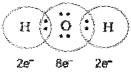
when two atoms share one electron pair they are said to be joined by a single covalent bond. If two atoms share two pairs of electrons, the covalent bond between them is called a double bond. And when combining atoms share three electron pairs as in the case of N2 molecule a triple bond a triple bond is formed.
- The total number of electrons required for writing the structures are obtained by adding the valence electrons of the combining atoms.
- For anions, each negative charge would mean addition of one electron. For cations, each positive charge would result in subtraction of one electron from the total number of valence electrons.
- The least electronegative atom occupies the central position in the molecule/ion.
Formal charge
Formal charge (F.C.) on an atom in a Lewis structure = total number of valence electrons in the free atom— total number of non bonding (lone pairjelectrons—(1/2) total number of bonding(shared)electrons.
Let us consider the ozone molecule (O3).
The Lewis structure of O3 may be drawn as:
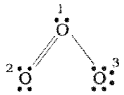
The atoms have been marked as 1,2 and 3. The formal charge on:
- The central O atom marked 1 = 6 – 2- \(\frac{1}{2}\)(6) = +1
- The end O atom marked 2 = 6 – 4 – \(\frac{1}{2}\)(4) = o
- The end O atom marked 3 = 6 – 6 – \(\frac{1}{2}\)(2) = -1
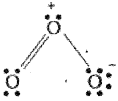
Hence, we represent O3 along with the formal changes as follows:
Limitations of the Octet Rule
There are three types of exceptions to the octet rule. The incomplete octet of the central atom In some compounds, the number of electrons surrounding the central atom is less than eight. This is especially the case with elements having less than four valence electrons.
Some compounds are BCl3, AlCl3 and BF3.
![]()
Odd-electron molecules
In molecules with an odd number of electrons like nitric oxide, NO and nitrogen dioxide, NO2, the octet rule is not satisfied for all the atoms.
![]()
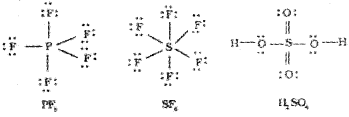
The expanded octet
In a number of compounds of these elements there are more than eight valence electrons around the central atom.Some examples are given below.
Ionic Or Electrovalent Bond
The formation of a positive ion involves ionization, i.e., removal of electrons from the neutral atom and that of the negative ion involves the addition of electron(s) to the neutral atom.
A qualitative measure of the stability of an ionic compound is provided by its enthalpy of lattice formation and not simply by achieving octet of electrons around the ionic species in gaseous state.
Lattice Enthalpy
The Lattice Enthalpy of an ionic solid is defined as the energy required to completely separate one mole of a solid ionic compound into gaseous constituentions.
Bond Parameters
Bond Length
It may be defined as the equilibrium distance between the centres of the nuclei of the two bonded i atoms in a molecule. Bond length are measured by spectroscopic, X-ray diffraction and electron diffraction techniques. It is usually expressed in Angstrom units (A°) or picometres (pm)
1 A° = 10-10m and 1 pm = 10-12m
Bond Angle
It is defined as the angle between the orbitals containing bonding electron pairs around the central atom in a molecule.
Bond Enthalpy
It is defined as the amount of energy required to break one mole of bonds of a particular type between two atoms in a gaseous state.
Bond Order:
In the Lewis description of covalent bond, the bond order is given by the number of bonds between the two atoms in a molecule. For example, the bond order in H2 is one, in O2 is two and in N2 is three. Isoelectronic molecules and ions have identical bond orders. For example N2, CO and NO+ have bond order 3. It is found that as the bond order increases, bond enthalpy increases and bond length decreases.
Resonance Structures
It is often observed that a single Lewis structure is inadequate for the representation of a molecule in conformity with its experimentally determined parameters. As in the case of O3.
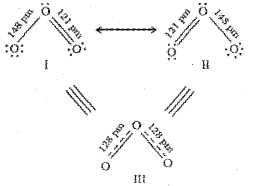
O3 is represented by the above 3 structures. These are called canonical structures.
Experimentally determined oxygen-oxygen bond lengths in the O3 molecule are same (128 pm). Thus the oxygen-oxygen bonds in the O3 molecule are intermediate between a double and a single bond. According to the concept of resonance, the canonical structures of the hybrid describes the molecule accurately.
Some of the other examples of resonance structures are provided by the carbonate ion and the carbon dioxide molecule.
Polarity of Bonds
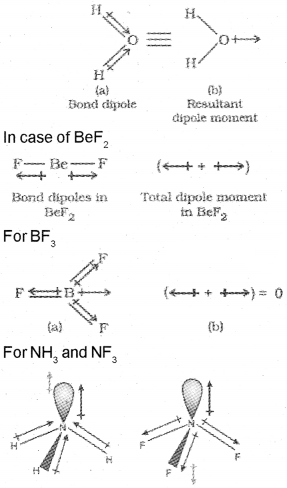
In reality no bond or a compound is either completely covalent or ionic. Even in case of covalent bond between two hydrogen atoms, there is some ionic character. As a result of polarisation, the molecule possesses the dipole moment. Which can be defined as the product of the magnitude of the charge and the distance between the centres of positive and negative charge. It is usually designated by a Greek letter Mathematically, it is expressed as follows: Dipole moment (µ) = change (Q) X distance of separation (r)
In case of polyatomic molecules, the dipole moment not only depend upon the individual dipole moments of bonds known as bond dipoles but also on the spatial arrangement of various bonds in the molecule.lt is due to the shifting of electrons to the side of more eletro negative element. For example,
![]()
The shifting of electrons is represented by an arrow. In case of H2O the resultant dipole moment is given by the following figure:
Fajans Rules:
Just as all the covalent bonds have some partial ionic character, the ionic bonds also have partial covalent character. The partial covalent character of ionic bonds was discussed by Fajans in terms of the following rules:
- The smaller the size of the cation and the larger the size of the anion, the greater the covalent character of an ionic bond.
- The greater the charge on the cation, the greater the covalent character of the ionic bond.
- For cations of the same size and charge, the one, with electronic configuration (n-1)d”ns°, typical of transition metals, is more polarising than the one with a noble gas configuration, ns2 np6, typical of alkali and alkaline earth metal cations.
The cation polarises the anion, pulling the electronic charge toward itself and thereby increasing the electronic charge between the two. This is precisely what happens in a covalent bond, i.e., buildup of electron charge density between the nuclei. The polarising power of the cation, the polarisability of the anion and the extent of distortion (polarisation) of anion are the factors, which determine the per cent covalent character of the ionic bond.
The Valence Shell Electron Pair Repul-Sion (Vspert) Theory
The main postulates of VSEPR theory are as follows:
- The shape of a molecule depends upon the number of valence shell electron pairs (bonded or nonbonded) around the central atom.
- Pairs of electrons in the valence shell repel one another since their electron clouds are negatively charged.
- These pairs of electrons tend to occupy such positions in space that minimise repulsion and thus maximise distance between them.
- The valence shell is taken as a sphere with the electron pairs localising on the spherical surface at maximum distance from one another.
- A multiple bond is treated as if it is a single electron pair and the two or three electron pairs of a multiple bond are treated as a single super pair.
- Where two or more resonance structures can represent a molecule, the VSEPR model is applicable to any such structure.
The repulsive interaction of electron pairs de-crease in the order:
Lone pair (lp) – Lone pair (lp) > Lone pair (lp) – Bond pair (bp) > Bond pair (bp) – Bond pair (bp)
Valence Bond Theory
Valence bond theory was introduced by Heitlerand London (1927) and developed further by Pauling and others. A discussion of the valence bond theory is based on the knowledge of atomic orbitals, electronic configurations of elements, the overlap criteria of atomic orbitals, the hybridization of atomic orbitals and the principles of variation and superposition. First, we consider the formation of H2. When the attractive forces become greater than the repulsive forces, the molecule is formed and the system gets minimum energy. Because energy is released when a bond is formed. The energy so released is called bond enthalpy.
Orbital Overlap Concept
When two atoms approach each other, their atomic orbitals undergo partial interpenetration. This partial interpenetration of atomic orbitals is called overlapping of atomic orbitals. The electrons belonging to these orbitals are said to be shared and this results in the formation of a covalent bond. The main ideas of orbital of overlap concept of formation of covalent bonds are
- Covalent bonds are formed by the overlapping of half filled atomic orbitals present in the valence shell of the atoms taking part in bonding.
- The orbitals undergoing overlapping must have electrons with opposite spins.
Overlapping of atomic orbitals results in decrease of energy and formation of covalent bond. - The strength of a covalent bond depends upon the extent of overlapping. The greater the overlapping, the stronger is the bond formed.
The above treatment of formation of covalent bond involving the overlap of half-filled atomic orbitals is called valence bond theory.
Types of Overlapping and Nature of Covalent Bonds
The covalent bond may be classified into two types depending upon the types of overlapping:
(i) Sigma(σ) bond, and
(ii) pi(π) bond
(i) Sigma( σ) bond :
This type of covalent bond is formed by the end to end (hand-on) overlap of bonding orbitals along the internuclear axis. This is called as head on overlap or axial overlap. This can be formed by any one of the following types of combinations of atomic orbitals.
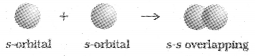
s-s overlapping:
In this case, there is overlap of two half filled s-orbitals along the internuclear axis as shown below:
s-p overlapping:
This type of overlap occurs between half filled s-orbitals of one atom and half filled p-orbitals of another atom.
![]()
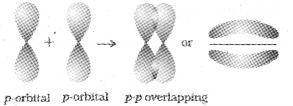
p-p overlapping :
This type of overlap takes place between half filled p-orbitals of the two approaching atoms.
![]()
(ii) pi(π) bond :
In the formation of n bond the atomic orbitals overlap in such a way that their axes remain parallel to each other and perpendicular to the internuclear axis. The orbitals formed due to side wise overlapping consists of two saucer type charged clouds above and below the plane of the participating atoms.
Strength of Sigma and pi Bonds
Basically the strength of a bond depends upon the extent of overlapping. In case of sigma bond, the overlapping of orbitals takes place to a larger extent. Hence, it is stronger as compared to the pi bond where the extent of overlapping occurs to a smaller extent. Further, it is important to note that pi bond between two atoms is formed in addition to a sigma bond. It is always present in the molecules containing multiple bond (double ortriple bonds).
Hybridisation
Hybridisationis defined as the process of intermixing of the orbitals of slightly different energies so as to redistribute their energies, resulting in the formation of new set of orbitals of equivalent energies and shape.
The number of hybrid orbitals is equal to the number of the atomic orbitals that get hybridised.
These hybrid orbitals are stable due to their arrangement which provides minimum repulsion between electron pairs. Therefore, the type of hybridisation indicates the geometry of the molecules.
It is not necessary that only half filled orbitals participate in hybridisation. In some cases, even filled orbitals of valence shell take part in hybridisation.
Types of Hybridisation
There are various types of hybridisation involving s, p and d orbitals. The different types of hybridisation are as under:
(I) sp hybridisation:
This type of hybridisation involves the mixing of one s and one p orbital resulting in the formation of two equivalent sp hybrid orbitals. Each sp hybrid orbitals has 50% s-character and 50% p-character. Such a molecule in which the central atom is sp- hybridised and linked directly to two other central atoms possesses linear geometry.The two sp hybrids point in the opposite direction which provides more effective overlapping resulting in the formation of stronger bonds.
Example of molecule having sp hybridisation BeCl2:
The ground state electronic.configuration of Be is 1s²2s². In the exited state one of the 2s-electrons is promoted to vacant 2p orbital to account for its divalency. One 2s and one 2p-orbitalsget hybridised to form two sp hybridised orbitals. These two sp hybrid orbitals are oriented in opposite direction forming an angle of 180°. Each of the sp hybridised orbital overlaps with the 2p-orbital of chlorine axially and form two Be-Cl sigma bonds.
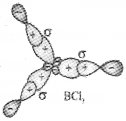
II) sp² hybridisation :
In this hybridisation there is involvement of one s and two p-orbitals in orderto form three equivalent sp² hybridised orbitals. For example, in BCl2 molecule, the ground state electronic configuration of central boron atom is 1s²2s²2p¹. In the excited state, one of the 2s
electrons is promoted to vacant 2p orbital as a result boron has three unpaired electrons.
These three orbitals (one 2s and two 2p) hybridise to form three sp2 hybrid orbitals. The three hybrid orbitals so formed are oriented in a trigonal planar arrangement and overlap with 2p orbitals of chlorine to form three B-Cl bonds. Therefore, in BCl3 the geometry is trigonal planar with ClBCl bond angle of 120°
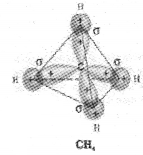
III) sp³ hybridisation:
This type of hybridisation can be explained by taking the example of CH4 molecule in which there is mixing of one s-orbital and three p-orbitals of the valence shell to form four sp³ hybrid orbital of equivalent energies and shape.
There is 25% s-character and 75% p-character in each sp³ hybrid orbital. The four sp3 hybrid orbitals so formed are directed towards the four corners of the tetrahedron.
The angle between sp³ hybrid orbital is 109.5°
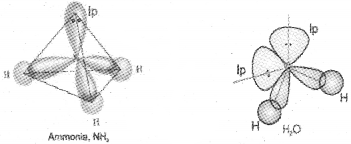
The structure of NH3 and H2O molecules can also be explained with the help of sp3hybridisation. In NH3, the valence shell (outer) electronic configuration of nitrogen in the ground state is 2s²\(p_{x}^{1} 2 p_{y}^{1} 2 p_{z}^{1}\) having three unpaired electrons in the sp³ hybrid orbitals and a lone pair of electrons is present in the fourth one. These three hybrid orbitals overlap with 1s orbitals of hydrogen atoms to form three N-H sigma bonds. Due to the force of repulsion, the molecule gets distorted and the bond angle is reduced to 107° from 109.5°. The geometry of such a molecule will be pyramidal.
Other Examples of sp³, sp² and sp Hybridisation:
sp³ Hybridisation in C2H6 molecule:
In ethane molecule both the carbon atoms assume sp3 hybrid state. One of the four sp³ hybrid orbitals of carbon atom overlaps axially with similar orbitals of other atom to form sp³-sp³ sigma bond while the other three hybrid orbitals of each carbon atom are used in forming sp³-s sigma bonds with hydrogen atoms Therefore in ethane C-C bond length is 154 pm and each C-H bond length is 109 pm.
sp² Hybridisation in C2H4:
In the formation of ethene molecule, one of the sp² hybrid orbitals of carbon atom overlaps axially with sp² hybridised orbital of another carbon atom to form C-C sigma bond. While the other two sp² hybrid orbitals of each carbon atom are used for making sp²-s sigma bond with two hydrogen atoms. The unhybridised orbital (2px or 2py) of one carbon atom overlaps sidewise with the similar orbital of the other carbon atom to form weak π bond, which consists of two equal electron clouds distributed above and below the plane of carbon and hydrogen atoms.
Thus, in ethene molecule, the carbon-carbon bond consists of one sp²-sp² sigma bond and one pi (π) bond between p orbitals which are not used in the hybridisation and are perpendicular to the plane of molecule; the bond length is 134 pm. The C-H bond is sp^s sigma with bond length 108 pm. The H-C-H bond angle is 117.6° while the H-C-C angle is 121°.
sp Hybridisation in C2H2:
In the formation of ethyne molecule, both the carbon atoms undergo sp- hybridisation having two unhybridised orbital i.e., 2py and 2px. One sp hybrid orbital of one carbon atom overlaps axially with sp hybrid orbital of the other carbon atom to form C-C sigma bond, while the other hybridised orbital of each carbon atom overlaps axially with the half filled s orbital of hydrogen atoms forming σ bonds. Each of the two unhybridised p orbitals of both the carbon atoms overlaps sidewise to form two π bonds between the carbon atoms. So the triple bond between the two carbon atoms is made up of one sigma and two pi bonds
Hybridisation of Elements involving d-Orbitals
The elements present in the third period contain d orbitals in addition to s and p orbitals. The energy of the 3d orbitals are comparable to the energy of the 3s and 3p orbitals. The energy of 3d orbitals are also comparable to those of 4s and 4p orbitals. As a consequence the hybridisation involving either 3s, 3p, and 3d or 3d, 4s and 4p is possible. However, since the difference in energies of 3p and 4s orbitals is significant, no hybridisation involving 3p, 3d and 4s orbitals is possible.
1. Formation of PCl5 (sp³d hybridisation):
The ground state and the excited state outer electronic configurations of phosphorus (Z=15) are represented below.
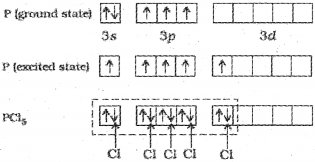
Now the five orbitals (i.eone s, three p, and one d orbitals) are available for hybridisation to yield a set of five sp3d hybrid orbitals which are directed towards the five comers of a trigonal bipyramidal.
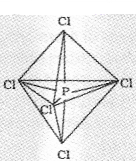
Three sigma bond known as equatorial bonds lie in one plane and make an angle of 120° with each other.
The remaining two P-Cl bonds(called axial bonds)-one lying above and the other lying below the equatorial plane, make an angle of 90° with the plane.
As the axial bond pairs suffer more repulsive interaction from the equatorial bond pairs, therefore axial bonds have been found to be slightly longer and hence slightly weaker than the equatorial bonds; which makes PCl5 molecule more reactive.
2. Formation of SF6 (sp³d² hybridisation):
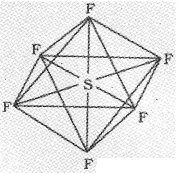
In SF6 the central sulphur atom has the ground state outer electronic configuration 3s²3p4. In the exited state the available six orbitals i.e., one s, three p and two d are singly occupied by electrons. These orbitals hybridise to form six new sp³d² hybrid orbitals, which are projected towards the six corners of a regular octahedron in SF6. These six sp³d² hybrid orbitals overlap with singly occupied orbitals of fluorine atoms to form six S-F sigma bonds. Thus SF6 molecule has a regular octahedral geometry.
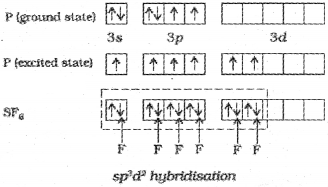
The structure of SF6 is given below.
Molecular Orbital Theory
Molecular orbital (MO) theory was developed by F. Hund and R.S. Mulliken in 1932. The salient features of this theory are :
- The electrons in a molecule are present in the various molecular orbitals as the electrons of atoms are present in the various atomic orbitals.
- The atomic orbitals of comparable energies and proper symmetry combine to form molecular orbitals.
- While an electron in an atomic orbital is influenced by one nucleus, in a molecular orbital it is influenced by two or more nuclei depending upon the number of atoms in the molecule. Thus, an atomic orbital is monocentric while a molecular orbital is polycentric.
- The number of molecular orbital formed is equal to the number of combining atomic orbitals. When two atomic orbitals combine, two molecular orbitals are formed. One is known as bonding molecular orbital while the other is called antibonding molecular orbital.
- The bonding molecular orbital has lower energy and hence greater stability than the corresponding antibonding molecular orbital.
- Just as the electron probability distribution around a nucleus in an atom is given by an atomic orbital, the electron probability distribution around a group of nuclei in a molecule is given by a molecular orbital.
- The molecular orbitals like atomic orbitals are filled in accordance with the aufbau principle obeying the Pauli’s exclusion principle and the Hund’srule.
Formation of Molecular Orbitals
Linear Combination of Atomic Orbitals (LCAO)
The atomic orbitals of these atoms may be represented by the wave functions ψA and ψB. The formation of molecular orbitals is the linear combination of atomic orbitals that can take place by addition and by subtraction of wave functions of individual atomic orbitals as shown below.
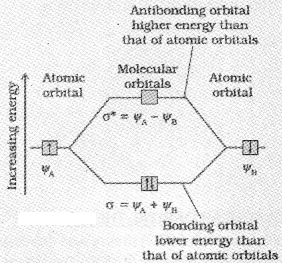
Energy Level Diagram for Molecular orbitals
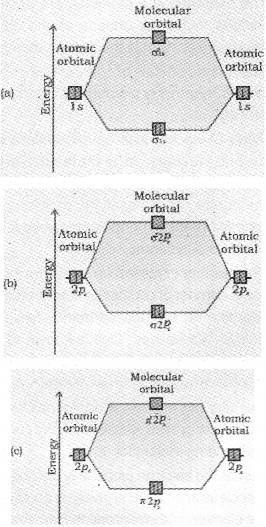
The increasing order of energies of various molecular orbitals for O2 and F2 is given below:
σ1s < σ*1s < σ2s < σ*2s < σ2px <(π2px = π2py) <(π*2px = π*2pz) < σ*2px
This sequence of energy levels of molecular orbitals is not correct for the remaining molecules Li2, Be2, B2, C2, N2. For molecules such as B2, C2, N2 etc. the increasing order of energies of various molecular orbitals is
σ1s < σ*1s < σ2s < σ*2s <(π2px = π2py) < σ2px <(π*2px = π*2pz) < σ*2pz
The important characteristic feature of this order is that the energy of σ 2pz molecular orbital is higher than that of π2px and π2py molecular orbitals. If the bonding influence is stronger a stable molecule results and if the antibonding influence is stronger,the molecule is unstable.
Bonding In Some Homonuclear Diatomic Molecules
Bond Order:
Bond order is defined as half of the difference between the number of electrons in the bonding molecular orbitals and that in the antibonding molecular orbitals.
Nh – N
i.e. Bond Order= \(\frac{N_{b}-N_{a}}{2}\). Where Nb is the number of electrons in the bonding molecular orbitals and Na is the number of electrons in the antibonding mo-lecular orbitals.
Significance of bond order:
Bond order conveys the following important informations about a molecule.
i) If the value of bond order is positive, it indicates a stable molecule and if the value of bond order is negative or zero, the molecule is unstable and is not formed.
ii) Bond dissociation energy of a diatomic molecule is directly proportional to the bond order of the molecule. The greater the bond order, the higher is the bond dissociation energy.
iii) Bond order is inversely proportional to the bond length. The higherthe bond ondervalue, smaller is the bond length. For example, the bond length in N2 molecule (having bond order 3) is less than that in O2 molecule (having bond order 2).
Magnetic character:
If all the electrons in the mol-ecules of a substance are paired, the substance will be diamagnetic. On the other hand, if there are un-paired electrons in the molecule, the substance will be paramagnetic.
Hydrogen Bonding
Hydrogen bond can be defined as the attractive force which binds hydrogen atom of one molecule with the electronegative atom (F, O orN) of another molecule. When hydrogen is bonded to strongly electronegative element ‘X’, the electron pair shared between the two atoms moves far away from hydrogen atom. As a result the hydrogen atom becomes, highly electropositive with respect to the other atom ‘X’. Since there is displacement of electrons towards X, the hydrogen acquires fractional positive charge (δ+) while ‘X’ attain fractional negative charge (δ–). This results in the formation of a polar molecule having electrostatic force of attraction which can be represented as: Hδ+ – Xδ-
The magnitude of H-bonding depends on the physical state of the compound. It is maximum in the solid state and minimum in the gaseous state. Thus, the hydrogen bonds have strong influence on the structure and properties of the compounds.
Types of Hydrogen Bonds
There are two types of hydrogen bonds
- Intermolecular hydrogen bond
- Intramolecular hydrogen bond
1. Intermolecular hydrogen bond:
It is formed between two different molecules of the same or different compounds. For example, H-bond in case of HF molecule, alcohol or water molecules, etc.
2. Intramolecular hydrogen bond:
It is formed when hydrogen atom is in between the two highly electronegative (F, O, N) atoms present within the same molecule. For example, in o-Nitrophenol the hydrogen is in between the two oxygen atoms as shown below:
