Kerala Plus One Chemistry Notes Chapter 12 Organic Chemistry: Some Basic Principles and Techniques
Introduction
The element carbon organic chemistry the element carbon has the unique property called catenation due to which it forms covalent bonds with other carbon atoms. It also forms covalent bonds with atoms of other elements like hydrogen, oxygen, nitrogen, sulphur, phosphorus and halogens. The resulting compounds are studied under a separate branch of chemistry called organic chemistry.
General Introduction
In early years of chemistry, compounds were classified into two types. Compounds derived from non-living sources such as rocks, minerals etc. were called ‘inorganic compounds’ and those derived from plants and animals were regarded as ‘organic compounds’. On account of the special nature of organic compounds and their occurrence in living world alone, it was believed that they were produced by a vital force existing in living organisms. This led to the belief that such compounds could not be synthesised in the laboratory. However in 1828, F. Wohler succeeded in preparing urea (an organic compound) from an inorganic material, ammonium cyanate.
Tetravalance Of Carbon
Shapes of Organic Compounds
Carbon (atomic number 6) has the ground state electronic configuration 1 s² 2s²p¹x2p¹y2p°z. Carbon attains noble gas configuration only by sharing electrons with other atoms. Carbon atom, therefore, forms four covalent bonds in all its compounds.
During the formation of bonds (which is an energy releasing process) the two electrons in the 2s orbital get unpaired and one is promoted to the empty 2pz orbital. This corresponds to the excited state of carbon which has four half-filled orbitals (four valence electrons). The shapes of molecules such as methane (CH4), ethene (C2H4) and ethyne (C2H2) are explained in terms of the use of sp³, sp² and sp hybridised orbitals by the carbon atoms in the respective molecules.
Structural Representations Of Organic Compounds
Complete, Condensed and Bond-line Structural Formulas
Structures of organic compounds are represented in several ways. The Lewis structure or dot structure, dash structure, condensed structure and bond line structural formulas are some of the specific types. In Lewis structures bonds are represented by lines and lone pair electrones by dots. For example,
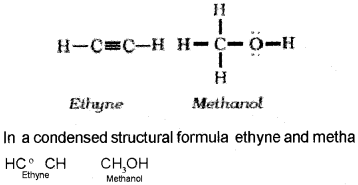
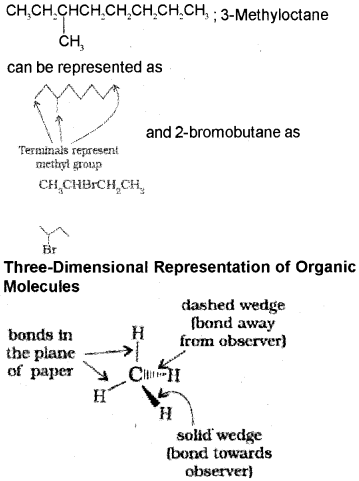
In bond-line structural representation of organic compounds, carbon and hydrogen atoms are not shown and the lines representing carbon-carbon bonds are drawn in a zig-zag fashion. The only atoms specifically written are oxygen, chlorine, nitrogen etc. The terminals denote methyl (-CH3) groups.
Classification Of Organic Compounds
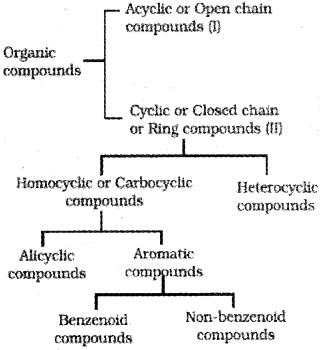
I. Acyclic or open chain compounds
These compounds are also called as aliphatic compounds and consist of straight or branched chain compounds, for example:
II. Alicyclic or closed chain or ring compounds
Alicyclic (aliphatic cyclic) compounds contain carbon atoms joined in the form of a ring (homocyclic). Sometimes atoms other than carbon are also present in the ring (heterocyclic). Some examples of this type of compounds are:
CycloiTexane Tetrahydrofuran These exhibit some of the properties similar to those of aliphatic compounds ane:
Aromatic compounds
Aromatic compounds are special types of compounds. You will learn about these compounds in detail in Unit 13. These include benzene and other related ring compounds (benzenoid). Like alicyclic compounds, aromatic comounds may also have hetero atom in the ring. Such compounds are called heterocyclic aromatic compounds. Some of the examples of various types of aromatic compounds are:
Benzenoid aromatic compounds
Non-benzenoid compound
Heterocyclic aromatic compounds
Organic compounds can also be classified on the basis of functional groups, into families or homologous series.
Homologous series
Homologous series may be defined as a series of similarly constituted organic compound in which the members possess the same functional group and have similar chemical properties and the neighbouring (or consecutive) members differ by – CH2 unit in their molecular formula,
eg: Alkane family (Cn H2n+2), alkenes (CnH2n) alcohols (Cn H2n+1OH).
Nomenclature Of Organic Compounds
IUPAC names of unbrached saturated hydrocarbons
Nomenclature of branched chain alkanes:
We encounter a number of branched chain alkanes. The rules for naming them are given below.
1. First of all, the longest carbon chain in the molecule is identified.For example,
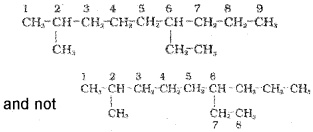
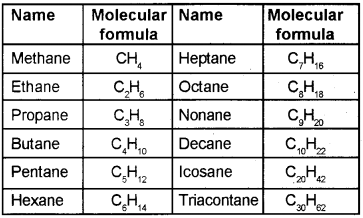
2. The numbering is done in such a way that the branched carbon atoms get the lowest possible numbers.
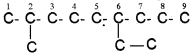
and the numbering is not from right to left.
3. The names of alkyl groups attached as a branch are then prefixed to the name of the parent alkane and position of the substituents is indicated by the appropriate numbers. If different alkyl groups are present, they are listed in alphabetical order. Thus, name for the compound shown above is: 6- Ethyl-2- methylnonane.
[Note: the numbers are separated from the groups by hyphens and there is no break between methyl and nonane.]
4. If two or more identical substituent groups are present then the numbers are separated by commas. The names of identical substituents are not repeated, instead prefixes such as di (for 2), tri (for 3), tetra (for 4), Penta (for 5), Hexa (for 6) etc. are used. While writing the name of the substituents in alphabetical order, these prefixes, however, are not considered. Thus, the following compounds are named as:
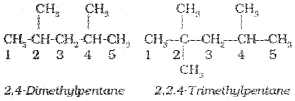
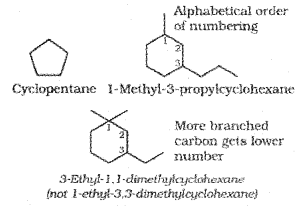
5. If the two substituents are found in equivalent positions, the lower number is given to the one coming first in the alphabetical listing. Thus, the following compound is 3-Ethyl-6-methyl octane and ‘ not6-Ethyl-3-methyl octane.
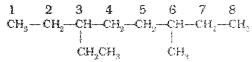
6. The branched alkyl groups can be named by following the above mentioned procedures. However, the carbon atom of the branch that attaches to the root alkane is numbered 1 as exemplified below.
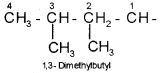
Cyclic Compounds
A saturated monocyclic compound is named by prefixing ‘cyclo’ to the corresponding straight chain alkane. If side chains are present, then the rules given above are applied. Names of some cyclic compounds are given below.
Nomenclature Of Organic Compounds Having Functional Group (S)
When a functional group (other than C=C and -C ≡ C) is present in the molecule, it is indicated by adding secondary suffix after the primary suffix. The terminal ‘e’ of the primary suffix is removed before adding secondary suffix (whose name begins with a, i, o, u or y). It is to be noted that some functional group such as alkoxy (-OR), nirto (-NO2), halogeno etc. are indicated by the prefixes.
For example, CH3-CH2-OH Ethane -e+ol = Ethanol (using secondary suffix) CH3-CH2-CHO Propane -e+al = Propanal (using secondary suffix) CH3-CH2-NO2 Nitroethane (using prefix)
The systematic name of an organic compound containing functional group can be derived using the following sequence of steps.
The longest carbon chain (parent chain) containing the functional groups is identified. This gives the word root. The name of the compound is then obtained as follows:
Prefixes – word root – primary suffix – secondary suffix The following examples will illustrate the rules
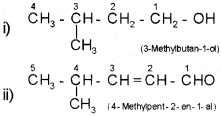
In case of compounds containing more than one similar functional group, the world di, tri etc is added before the secondary suffix which indicates the functional group. In doing so, the last letter ‘e’ of the parent alkane has to be retained. However, the endingne of the parent alkane is dropped in case of compounds having more than one double or triple bond. For example,
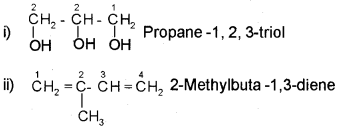
If the molecule contains two or more different functional groups, the parent chain must contain maximum possible number of functional groups. The carbon atoms in the parent chain are numbered in such a way that the functional group of higher priority gets the lower number. The priority of various functional groups follows the order.
COOH>-CO-O-CO->-COOR>-COCI>-CONH2>-CN>- HC=O>CO>-OH>-NH2>>C=C<-C≡C->-X>-NO2>R-
The functional group with higher priority is indicated by suitable secondary suffix and the other functional groups are treated as substituents which are specified by suitable prefixes.
For example,
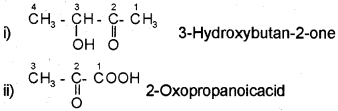
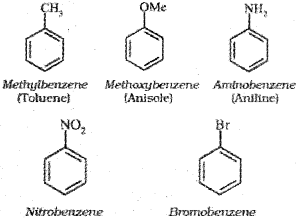
Nomenclature of Substituted Benzene Compounds
For IUPAC nomenclature of substituted benzene compounds, the substituent is placed as prefix to the word benzene as shown in the following examples.
If benzene ring is disubstituted, the position of substituents is defined by numbering the carbon atoms of the ring such that the substituents are located at the lowest numbers possible. For example, the compound(b) is named as 1, 3-Dibromobenzene and not as 1,5 dibromobenzene. Substituent of the base compound is assigned number 1 and then the direction of numbering is chosen such that the next substituent gets the lowest number. The substituents appear in the name in alphabetical order. Some examples are given below.
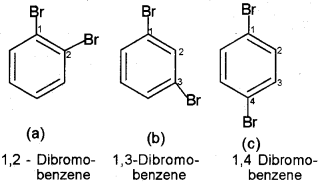
In the trivial system of nomenclature the terms ortho (o), meta (m) and para (p) are used as prefixes to indicate the relative positions 1,2-;1,3- and Ir-respectively. Thus, 1,3-dibromobenzene (b) is named as m-dibromobenzene (meta is abbreviated as m-) and the other isomers of dibromobenzenel, 2-(a) and 1,4-(c), are named as ortho (or just o-) and para (or just p-)-dibromobenzene, respectively. The substituents appear in the name in alphabetical order.
Isomerism
The phenomenon of existence of two or more compounds possessing the same molecular formula but different properties is known as isomerism. Such compounds are called as isomers.
Structural Isomerism
Compounds having the same molecular formula but different structures (manners in which atoms are linked) are classified as structural isomers. Some typical examples of different types of structural isomerism are given below:
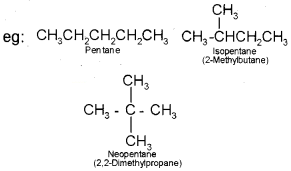
(i) Chain isomerism:
When two or more compounds have similar molecular formula but different carbon skeletons, these are referred to as chain isomers and the phenomenon is termed as chain isomerism.
ii) Position isomerism:
When two or more compounds differ in the position of substituent atom or functional group on the carbon skeleton, they are called position isomers and this phenomenon is termed as position isomerism.
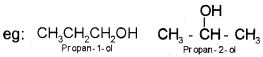
iii) Functional group isomerism :
Two or more compounds having the same molecular formula but different functional groups are called functional isomers and this phenomenon is termed as functional group isomerism.
iv) Metamerism :
It arises due to different alkyl chains on either side of the functional group in the molecule. For example, CaH10O represents Methoxypropane (CH3OC3H7) and Ethoxyethane (C2H5OC2H5).
Fundamental Concepts In Organic Reaction Mechanism
An organic reaction takes place by the attack of a reagent on an organic compound which is designated as a substrate. The steps of an organic reaction showing the breaking and formation of bonds in such substrate leading to the formation of the final product are referred to as its mechanism.
Fission Of A Covalent Bond
A covalent bond can be broken in two different ways,
(i) Homolytic fission :
If a covalent bond breaks in such a way that each atom takes away one electron of the shared pair. It is called homolytic fission or homolysis. The fragments with odd or unpaired electrons formed by homolysis are known as free radicals. For example,
![]()
Usually homolysis occurs at high temperature or in presence of high energy radiations. Reactions occurring through homolytic fission are known as free radical reactions (non-polar reactions).
(ii) Heterolytic fission :
When a covalent bond breaks in such a way that both the electrons of the covalent bond are taken away by one of the bonded atoms, the mode of cleavage is called heterolytic cleavage or heterolysis. The products of heterolysis of a covalent bond are positive and negative ions, eg:
![]()
Inductive Effect (I Effect)
It is the permanent polarisation of a sigma bond in a molecule by the influence of an adjacent polar bond or group.
For illustration, let us consider a carbon chain in which one terminal carbon atom is joined to a chlorine atom. Since the chlorine atom is more electronegative than carbon, the sigma electrons of the C-Cl bond are displaced towards the chlorine atom. As a result, the chlorine atom acquires a small negative charge and C acquires a small positive charge as shown below. The magnitude of+charge is of the order C1 > C2 >C3.
![]()
This type of electron displacement of sigma electrons along a saturated carbon chain due to the presence of an electron-withdrawing group (or electron-donating group) is called Inductive effect:
This effect decreases sharply with increasing distance from the substituent and becomes negligible afterthe third carbon in a chain. Atoms orgroups of atoms that attract or withdraw electrons from a chain are said to have electron withdrawing inductive effect or-l effect, eg:,
-NO2 >- CN >- COOH >- F >- Cl >- Br >-l
Atoms or groups which push or donate electrons to a carbon chain are said to have electron releasing inductive effect or + l effect. Alkyl groups have +l effect and the order of + l effect of alkyl groups is
![]()
Resonance Structure
There are many organic molecules whose behaviour cannot be explained by a single Lewis structure. An example is that of benzene. Its cyclic structure containing alternating C-C single and C=C double bonds shown is inadequate for explaining its characteristic properties.
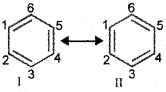
As per the above representation, benzene should exhibit two different bond lengths, due to C-C single and C=C double bonds. Thus, the structure of benzene cannot be represented adequately by the above structure. Further, benzene can be represented equally well by the energetically identical structures I and ll. The actual structure of benzene cannot be adequately represented by any of these structures, rather it is a hybrid of the two structures (I and II) called resonance structures.
The resonance structures are hypothetical and individually do not represent any real molecule. They contribute to the actual structure in proportion to their stability. The energy of actual structure of the molecule (the resonance hybrid) is lower than that of any of the canonical structures. The difference in energy is called the resonance stabilisation energy or simply the resonance energy. The more the number of important contributing structures, the more is the resonance energy. Resonance is particularly important when the contributing structures are equivalent in energy.
Resonance Effect
The resonance effect is defined as ‘the polarity produced in the molecule by the interaction of two π- bonds or between a π-bond and lone pair of electrons present on an adjacent atom’.The effect is transmitted through the chain. There are two types of resonance or mesomeric effect designated as R or M effect.
(i) Positive Resonance Effect (+R effect)
In this effect, the transfer of electrons is away from an atom or substituent group attached to the conjugated system. This electron displacement makes certain positions in the molecule of high electron densities.
(ii) Negative Resonance Effect (- R effect)
This effect is observed when the transfer of electrons is towards the atom or substituent group attached to the conjugated system.The atoms or substituent groups, which represent +R or-R electron displacement effects are as follows:
+R effect: – halogen, -OH, -OR, -OCOR, -NH2, – NHR,-NR2,-NHCOR,
– R effect: – COOH, -CHO, >C=O, – CN, -NO2
Electromeric Effect (E – Effect)
This is temporary effect which involves the complete transfer of n electrons of a multiple bond to one of the bonded atoms in presence of an attacking reagent. However, when the attacking reagent is removed, the polarised molecule shifts back to its original electronic condition.
With transfer of π electrons takes place towards the attacking reagent the effect is called +E effect and when the transfer of π electrons occurs-S away from the attacking reagent the effect is called -E effect.
Hyperconjugation
Hyperconjugation is a general stabilising interaction. It involves delocalisation of σ electrons of C—H bond of an alkyl group directly attached to an atom of unsaturated system or to an atom with an unshared p orbital. The σ electrons of C—H bond of the alkyl group enter into partial conjugation with the attached unsaturated system or with the unshared p orbital. Hyperconjugation is a permanent effect.
Methods Of Purification Of Organic Compounds
Sublimation:
On heating, some solid substances change from solid to vapour state without passing through liquid state. The purification technique based on the above principle is known as sublimation and is used to separate sublimable compounds from nonsublimable impurities.
Crystallisation:
It is based on the difference in the solubilities of the compound and the impurities in a suitable solvent. The impure compound is dissolved . in a solvent in which it is sparingly soluble at room temperature but appreciably soluble at higher temperature. The solution is concentrated to get a nearly saturated solution. On cooling the solution, pure compound crystallises out and is removed by filtration. The filtrate (mother liquor) contains impurities and small quantity of the compound.
Distillation:
This important method is used to separate (i) volatile liquids from non-volatile impurities and (ii) the liquids having sufficient difference in their boiling points. Liquids having different boiling points vaporise at different temperatures. The vapours are cooled and the liquids so formed are collected separately. Chloroform (b.p 334 K) and aniline (b.p. 457 K) are easily separated by the technique of distillation. On boiling, the vapours of lower boiling component are formed first. The vapours are condensed by using a condenser and the liquid is collected in a receiver. The vapours of higher boiling component form later and the liquid can be collected separately.
Fractional Distillation:
If the difference in boiling points of two liquids is not much, simple distillation cannot be used to separate them. The vapours of such liquids are formed within the same temperature range and are condensed simultaneously. The technique of fractional distillation is used in such cases. In this technique, vapours of a liquid mixture are passed through a fractionating column before condensation. The fractionating column is fitted over the mouth of the round bottom flask. Vapours of the liquid with higher boiling point condense before the vapours of the liquid with lower boiling point. The vapours rising up in the fractionating column become richer in more volatile component. By the time the vapours reach to the top of the fractionating column, these are rich in the more volatile component. The vapours become richer in low boiling component. On reaching the top, the vapours become pure in low boiling component and pass through the condenser and the pure liquid is collected in a receiver.
After a series of successive distillations, the remaining liquid in the distillation flask gets enriched in high boiling component. Each successive condensation and vaporisation unit in the fractionating column is called a theoretical plate. One of the technological applications of fractional distillation is to separate different fractions of crude oil in petroleum industry. Distillation under reduced pressure: This method is used to purify liquids having very high boiling points and those, which decompose at or below their boiling points. Such liquids are made to boil at a temperature lower than their normal boiling points by reducing the pressure on their surface. A liquid boils at a temperature at which its vapour pressure is equal to the external pressure. The pressure is reduced with the help of a water pump or vacuum pump. Glycerol can be separated from spent-lye in soap industry by using this technique.
Steam Distillation:
This technique is applied to separate substances which are steam volatile and are immiscible with water. In steam distillation, steam from a steam generator is passed through a heated flask containing the liquid to be distilled. The mixture of steam and the volatile organic compound is condensed and collected. The compound is later separated from water using a separating funnelAniline is separated by this technique from aniline-water mixture.
Differential Extraction:
When an organic compound is present in an aqueous medium, it is separated by shaking it with an organic solvent in which it is more soluble than in water. The organic solvent and the aqueous solution should be immiscible with each other so that they form two distinct layers which can be separated by separatory funnel. The organic solvent is later removed by distillation or by evaporation to get back the compound.
Chromatography:
The name chromatography is based on the Greek word chroma, for colour since the method was first used for the separation of coloured substances found in plants. In this technique, the mixture of substances is applied onto a stationary phase, which may be a solid ora liquid.
A pure solvent, a mixture of solvents, or a gas is allowed to move slowly over the stationary phase. The components of the mixture get gradually separated from one another. The moving phase is called the mobile phase. Based on the principle involved, chromatography is classified into different categories. Two of these are:
- Adsorption chromatography, and
- Partition chromatography.
1. Adsorption Chromatography:
Adsorption chromatography is based on the fact that different compounds are adsorbed on an adsorbent to different degrees. Commonly used adsorbents are silica gel and alumina. When a mobile phase is allowed to move over a stationary phase (adsorbent), the components of the mixture move by varying distances over the stationary phase. Following are two main types of chromatographic techniques based on the principle of differential dsorption.
- Column chromatography, and
- Thin layer chromatography.
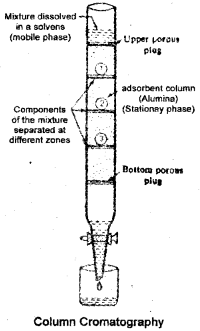
Column Chromatography
Column chromatography involves separation of a mixture overa column of adsorbent (stationary phase) packed in a glass tube. The column is fitted with a stopcock at its lower soluble in the organic solvent. The mixture adsorbed on adsorbent is placed on the top of the adsorbent column packed in a glass tube. An appropriate eluant which is a liquid or a mixture of liquids is allowed to flow down the column slowly. Depending upon the degree to which the compounds are adsorbed, complete separation takes place. The most readily adsorbed substances are retained near the top and others come down to various distances in the column.
Thin-layer chromatography (TLC) is another type of adsorption chromatography, which involves separation of substances of a mixture over a thin layer of an adsorbent coated on glass plate. A thin layer of an adsorbent (silica gel or alumina) is spread overa glass plate of suitable size. The plate is known as thin layer chromatography plate or chrome plate. The solution of the mixture to be separated is applied as a small spot about 2 cm above one end of the TLC plate. The glass plate is then placed in a closed jar containing the eluant. As the eluant rises up the plate, the components of the mixture move up along with the eluant to different distances depending on their degree of adsorption and separation takes place. The relative adsorption of each component of the mixture is expressed in terms of its retardation factor i.e. Rf value
Rf = x/y
Where distance moved by the substance from base line is x and distance moved by the solvent from base line is y. The spots of coloured compounds are visible on TLC plate due to their original colour. Another detection technique is to place the plate in a covered jar containing a few crystals of iodine. Spots of compounds, which adsorb iodine, will show up as brown spots. Sometimes an appropriate reagent may also be sprayed on the plate. For example, amino acids may be detected by spraying the plate with ninhydrin solution.
Partition Chromatography:
Partition chromatography is based on continuous differential partitioning of components of a mixture between stationary and mobile phases. Paper chromatography is a type of partition chromatography. In paper chromatography, a special quality paper known as chromatography paper is used. Chromatography paper contains water trapped in it, which acts as the stationary phase. A strip of chromatography paper spotted at the base with the solution of the mixture is suspended in a suitable solvent ora mixture of solvents. This solvent acts as the mobile phase. The solvent rises up the paper by capillary action and flows over the spot. The paper selectively retains different components according to their differing partition in the two phases. The paper strip so developed is known as a chromatogram. The spots of the separated coloured compounds are visible at different heights from the position of initial spot on the chromatogram. The spots of the separated colourless compounds may be observed either under ultraviolet light or by the use of an appropriate spray reagent as discussed under thin layer chromatography.
Qualitative Analysis Of Organic Compounds
Thin layer chromatography (TLC) is another type of adsorption chromatography, which involves separation of substances of a mixture over a thin layer of an adsorbent coated on glass plate. A thin
The elements present in organic compounds are carbon and hydrogen. In addition.to these, they may also contain oxygen, nitrogen, sulphur, halogens and phosphorus.
Detection of Carbon and Hydrogen
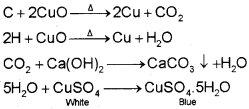
Carbon and hydrogen are detected by heating the compound with copper(ll) oxide. Carbon present in the compound is oxidised to carbon dioxide (tested with lime-water, which develops turbidity) and hydrogen to water (tested with anhydrous copper sulphate, which turns blue).
Detection of Other Elements
Nitrogen, sulphur, halogens and phosphorus present in an organic compound are detected by “Lassaigne’s test”. The elements present in the compound are converted from covalent form into the ionic form by fusing the compound with sodium metal. Following reactions take place:
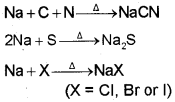
C, N, Sand X come from organic compound. Cyanide, sulphide and halide of sodium so formed on sodium fusion are extracted from the fused mass by boiling it with distilled water. This extract is known as sodium fusion extract.
1. Test for Nitrogen
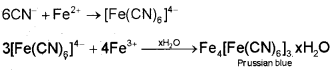
The sodium fusion extract is boiled with iron(ll) sulphate and then acidified with concentrated sulphuric acid. The formation of Prussian blue colour confirms the presence of nitrogen. Sodium cyanide first reacts with iron(ll) sulphate and forms sodium hexacyanoferrate(ll). On heating, with concentrated sulphuric acid some iron(ll) ions are oxidised to iron(lll) ions which react with sodium hexacyanoferrate(ll) to produce iron(lll) hexacyanoferrate(ll) (ferric ferrocyanide) which is Prussian blue in colour.
2. Test for Sulphur
1. The sodium fusion extract is acidified with acetic acid and lead acetate is added to it. A black precipitate of lead sulphide indicates the presence of sulphur.
![]()
2. On treating sodium fusion extract with sodium nitroprusside, appearance of a violet colour further indicates the presence of sulphur.
![]()
In case, nitrogen and sulphur both are present in an organic compound, sodium thiocyanate is formed. It gives blood-red colour and no Prussian blue since there are no free cyanide ions.
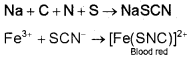
If sodium fusion is carried out with excess of sodium, the thiocyanate decomposes to yield cyanide and sulphide. These ions give their usual tests.
NaSCN + 2Na → NaCN + Na2S
3. Test for Halogens
The sodium fusion extract is acidified with nitric acid and then treated with silver nitrate. A white precipitate, soluble in ammonium hydroxide shows the presence of chlorine, a yellowish precipitate, sparingly soluble in ammonium hydroxide shows the presence of bromine and a yellow precipitate, insoluble in ammonium hydroxide shows the presence of iodine.
X– + Ag+ → AgX
X represents a halogen – Cl, Br or I. If nitrogen or sulphur is also present in the compound, the sodium fusion extract is first boiled with concentrated nitric acid to decompose cyanide or sulphide of sodium formed during Lassaigne’s test. These ions would otherwise interfere with silver nitrate test for halogens.
4. Test for Phosphorus
The compound is heated with an oxidising agent (sodium peroxide). The phosphorus present in the compound is oxidised to phosphate. The solution is boiled with nitric acid and then treated with ammonium molybdate. A yellow colouration or precipitate indicates the presence of phosphorus.
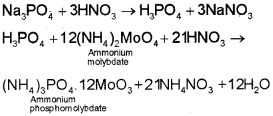
Quantitative Analysis
The percentage composition of elements present in an organic compound is determined by the methods based on the following principles:
Carbon and Hydrogen
Both carbon and hydrogen are estimated in one experiment. A known mass of an organic compound is burnt in the presence of excess of oxygen and copper(ll) oxide. Carbon and hydrogen in the compound are oxidised to carbon dioxide and water respectively.
CxHy + (x + y /4)O2 → xCO2 +(y/2)H2O
The mass of water produced is determined by passing the mixture through a weighed U-tube containing anhydrous calcium chloride. Carbon dioxide is absorbed in another U-tube containing concentrated solution of potassium hydroxide. These tubes are connected in series. The increase in masses of calcium chloride and potassium hydroxide gives the amounts of water and carbon dioxide from which the percentages of carbon and hydrogen are calculated. Let the mass of organic compound be m g, mass of water and carbon dioxide produced be m1 and m2g respectively;
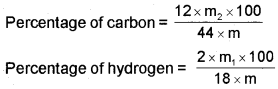
Nitrogen
There are two methods for estimation of nitrogen: (i) Dumas method and (ii) Kjeldahl’s method.
(i) Dumas method:
The nitrogen containing organic compound, when heated with copper oxide in an atmosphere of carbon dioxide, yields free nitrogen in addition to carbon dioxide and water.
CxHyNz+(2x + y/2)CuO → x CO2 + y / 2H2O +Z / 2N2 + (2x + y / 2)CU
Traces of nitrogen oxides formed, if any, are reduced to nitrogen by passing the gaseous mixture over a heated copper gauze. The mixture of gases so produced is collected over an aqueous solution of potassium hydroxide which absorbs carbon dioxide. Nitrogen is collected in the upper part of the graduated tube. Let the mass of organic compound = m g Volume of nitrogen collected = V1 mL
Room temperature = T1K
Volume of nitrogen at STP = 7\(\frac{p_{1} v_{1} \times 273}{760 \times T_{1}}\) (LetitbeVmL)
Where p1 and V1 are the pressure and volume of nitrogen, p,is different from the atmospheric pressure at which nitrogen gas is collected. The value of pt is obtained by the relation;
p1 Atmospheric pressure-Aqueous tension 22400 mL N2 at STP weighs 28 g.
\frac{p_{1} v_{1} \times 273}{760 \times T_{1}}
(ii) Kjeldahl’s method:
The compound containing nitrogen is heated with concentrated sulphuric acid. Nitrogen in the compound gets converted to ammonium sulphate. The resulting acid mixture is then heated with excess of sodium hydroxide. The liberated ammonia gas is absorbed in an excess of standard solution of sulphuric acid. The amount of ammonia produced is determined by estimating the amount of sulphuric acid consumed in the reaction. It is done by estimating unreacted sulphuric acid left after the absorption of ammonia by titrating it with standard alkali solution. The difference between the initial amount of acid taken and that left after the reaction gives the amount of acid reacted with ammonia.
taken = V mL
Volume of NaOH of molarity, M. used for titration of excess of H2SO4 = V1 mL
V1L of NaOH of molarity M= V1 /2 mL of H2SO4 0f molarity M
Volume of H2SO4 of molarity M unused= (V-1/1/2) mL (V-1/1/2) mL of H2S04 of molarity M = 2(V-V1/2) mL of NH3 solution of molarity M.
1000 mL of 1 M NH3 solution contains 17g NH3 or 14 g of N
2(V-V1/2) mL of NH3 solution of molarity M contains:
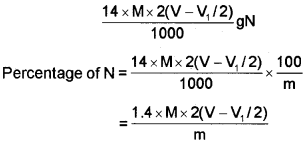
Kjeldahl method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in the ring (e.g. pyridine) as nitrogen of these compounds does not change to ammonium sulphate under these conditions.
Halogens
Carius method: A known mass of an organic compound is heated with fuming nitric acid in the presence of silver nitrate contained in a hard glass tube known as Carius tube, in a furnace. Carbon and hydrogen present in the compound are oxidised to carbon dioxide and water. The halogen present forms the corresponding silver halide (AgX). It is filtered, washed, dried and weighed.
Let the mass of organic compound taken = m g
Mass of AgX formed = m, g
1 mol of AgX contains 1 mol of X
Mass of halogen in m1g of AgX
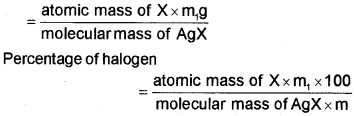
Sulphur
A known mass of an organic compound is heated in a Carius tube with sodium peroxide or fuming nitric acid. Sulphur present in the compound is oxidised to sulphuric acid. It is precipitated as barium sulphate by adding excess of barium chloride solution in water. The precipitate is filtered, washed, dried and weighed. The percentage of sulphur can be calculated from the mass of barium sulphate. Let the mass of organic compound taken = m g and the mass of barium sulphate formed = m1 g
1 mol of BaSO4 = 233 g BaSO4 = 32 g sulphur

Phosphorus
A known mass of an organic compound is heated with fuming nitric acid whereupon phosphorus present in the compound is oxidised to phosphoric acid. It is precipitated as ammonium phosphomolybdate, (NH4)3PO4.12MoO3, by adding ammonia and ammonium molybdate. Alternatively, phosphoric acid may be precipitated as MgNH4PO4 by adding magnesia mixture which on ignition yields Mg2P2O7. Let the mass of organic compound taken = m g and mass of ammonium phospho molydate = m1g.
Molar mass of (NH4)3PO4.12MoO3 = 1877 g.

where, 222 u is the molar mass of Mg2P2O7, m, the mass of organic compound taken, mv the mass of Mg2P2O7 formed and 62, the mass of two phosphorus atoms present in the compound Mg2P2O7.
Oxygen
The percentage of oxygen in an organic compound is usually found by difference between the total percentage composition (100) and the sum of the percentages of all other elements. However, oxygen can also be estimated directly as follows:
A definite mass of an organic compound is decomposed by heating in a stream of nitrogen gas. The mixture of gaseous products containing oxygen is passed over red-hot coke when all the oxygen is converted to carbon monoxide. This mixture is passed through warm iodine pentoxide (IJOJ) when carbon monoxide is oxidised to carbon dioxide producing iodine.
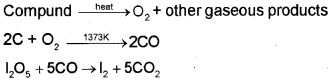
The percentage of oxygen can be derived from the amount of carbon dioxide or iodine produced.
Let the mass of organic = mg compund taken
Mass of carbon dioxide = m1g
44g carbon dioxide = 32 g oxygen
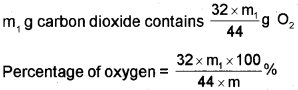
Presently, the estimation of elements in an organic compound is carried out by using microquantities of substances and automatic experimental techniques. The elements, carbon, hydrogen and nitrogen present in a compound are determined by an apparatus known as CHN elemental analyser. The analyser requires only a very small amount of the substance (1 -3 mg) and displays the values on a screen within a short time.
