Kerala Plus One Chemistry Notes Chapter 11 The p Block Elements
Introduction
There are six groups of p-block elements in the periodic table numbering from 13to 18. Boron, carbon, nitrogen, oxygen, fluorine and helium head the groups. Their valence shell electronic configuration is ns² np1-6(except for He). The inner core of the electronic configuration may, however, differ. The difference in inner core of elements greatly influences their physical properties (such as atomic and ionic radii, ionisation enthalpy, etc.) as well as chemical properties.
In groups 13, 14 and 15, the group oxidation state is the most stable state for lighter elements of the group. However, the oxidation state two units less than the group oxidation state becomes progressively more stable down a group. This is due to the reluctance of ns² electrons to participate in bond formation in the case of heavier elements. This phenomenon is known as inert pair effect. Since p-block contains non-metals (and metalloids), these elements have higher electronegativities and higher ionisation enthalpies. In contrast to metals which form cations, non-metals readily form anions.
The combined effect of size and availability of cf orbitals considerably influences the ability of these elements to form π bonds. The first member of a group differs from the heavier members in its ability to form pπ -pπ multiple bonds to itself ( e.g., C=C, C° C, N° N) and to other second row elements e.g., C=0, C=N, C° N, N=0). This type of π – bonding is not particularly strong for the heavier p-block elements. The heavier elements do form π bonds but this involves d orbitals.
Group 13 Elements: The Boron Family
Electronic Configuration
The outer electronic configuration of these elements is ns² np¹. This difference in electronic structures affects the other properties and consequently the chemistry of all the elements of this group.
Atomic Radii
On moving down the group, atomic radius is expected to increase. However, a deviation can be seen. Atomic radius of Ga is less than that of Al. This can be understood from the variation in the inner core of the electronic configuration. The presence of additional 10 d-electrons offer only poor screening effect for the outer electrons from the increased nuclear charge in gallium. Consequently, the atomic radius of gallium (135 pm) is less than that of aluminium (143 pm).
Ionization Enthalpy
The ionisation enthalpy values as expected from the general trends do not decrease down the group. The decrease from B to Al is associated with increase in size. The observed discontinuity in the ionisation enthalpy values between Al and Ga, and between In and Tl are due to inability of d- and f-electrons, which have low screening effect, to compensate the increase in nuclear charge.
Electronegativity
Down the group, electronegativity first decreases from B to Al and then increases marginally.
Physical Properties
Boron is non-metallic in nature. It is extremely hard and black coloured solid. It exists in many allotropic forms.
Chemical Properties
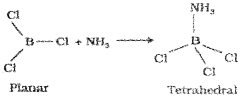
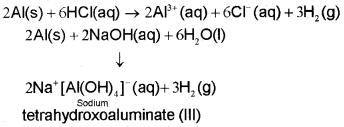
Oxidation state and trends in chemical reactivity The sum of its first three ionization enthalpies of boron is very high due to its small size. This prevents it to form +3 ions and forces it to form only covalent compounds. But as we move from BtoAl.the sum of the first three ionisation enthalpies of Al considerably decreases, and is, therefore, able to form Al3+ ions. The tendency to behave as Lewis acid decreases with the increase in the size down the group. BCl3 easily accepts a lone pair of electrons from ammonia to form BCl3.NH3.
i) Reactivity towards air
Boron has crystalline form which is unreactive. Alu-minium forms a very thin oxide layer on the surface which protects the metal from further attack.
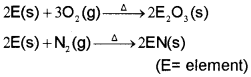
ii) Reactivity towards acids and alkalies
Boron does not react with acids and alkalies even at moderate temperature, but aluminium has amphoteric character.
iii) Reactivity towards halogens
2E(s) + 3X2(g) → 2EX3(S) (X = F, Cl, Br, I)
Important Trends And Anomalous Properties Of Boron
The tri-chlorides, bromides and iodides of all these elements being covalent in nature are hydrolysed in water. Species like tetrahedral [M(OH)4]– and octahedral [M(H2O)6]3+, except in boron, exist in aqueous medium. The monomeric trihalides, being electron deficient, are strong Lewis acids. Boron trifluoride easily reacts with Lewis bases such as NH3 to complete octet around boron.
F3B+: NH3 → F3B ← NH3
It is due to the absence of d orbitals that the maximum covalence of B is 4. Since the d orbitals are available with Al and other elements, the maximum covalence can be expected beyond 4. Most of the other metal halides (e.g., AlCl3 are dimerised through halogen bridging (e.g., Al2Cl6). The metal species ‘ completes its octet by accepting electrons from halogen in these halogen bridged molecules.
Some Important Compounds Of Boron
Borax
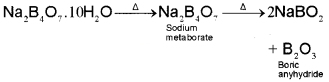
It is the most important compound of boron. Formula of the compound is Na2B4O7.10H2O . In fact it contains the tetranuclear units [B4O5(OH)4]2- and correct formula; therefore, is Na2[B4O5(OH)4].8H2O.
![]()
On heating, borax first loses water molecules. On further heating it turns into a transparent liquid, which solidifies into glass like material known as borax bead.
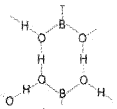
Orthoboric acid
Orthoboric acid, H3B03 is a white crystalline solid, with soapy touch. It is sparingly soluble in water but highly soluble in hot water.
Na2B4O7 + 2HCl + 5H2O → 2NaCl + 4B(OH)3
Boric acid is a weak monobasic acid. It is not a protonic acid but acts as a Lewis acid by accepting electrons from a hydroxyl ion:
B(OH)3 +2HOH → [B(OH)4]– + H3O+
Structure of boric acid is given below.
Diborane (B2H6)
The simplest boron hydride is diborane (B2H6). Diborane can be prepared by treating BF3 with lithium aluminium hydride in ether. A convenient laboratory method is oxidation of sodium borohydride with iodine.
2NaBH4 + l2 → B2H6 + 2Nal +H2
On a commercial scale, diborane is produced by the action of BF3 on sodium hydride.
![]()
Diborane is a colourless toxic gas. It catches fire on exposure to air releasing large amount of energy.
B2H6+ 6H2O → 2B(OH)3 + 6H2
Reaction of diborane with NH3 gives an addition product B2H6.2NH3 which on heating gives borazine (B3N3H3), commonly known as inorganic benzene due to its structural similarity with benzene. Boron forms a series of hydridoborates, the most important being (BH4)–.NaBH4 (sodium borohydride) is a good reducing agent.
Each boron atom in B2H6 is sp³ hybridised. The structure contains two types of H- atoms the four-terminal hydrogen atoms and two bridged hydrogen atoms. The four-terminal H atoms and two B atoms lie in the same plane. Above and below this plane lie the bridged H atoms. B-H bonds formed by the terminal hydrogen atoms are normal covalent bonds while the bridge B-H bonds are three centre two-electron bonds. Each B atom forms four bonds even though boron has only three valence electrons. Hence B2H6 is an electron deficient compound.
Group 14 Elements: The Carbon Family
Carbon, silicon, germanium, tin, and lead form the carbon family.
Occurrence:
Carbon is widely distributed in nature in the free and combined states. Graphite, diamond, coal, etc are elemental forms of carbon while in the combined state it occurs as metal carbonates, hydrocarbons and CO2 in air. Silicon is present in nature as silica and silicates. Ge is found only in traces. Tin occurs as cassiterite (SnO2) and lead as galena (PbS)
Electronic Configuration
The valence shell electronic configuration of these elements is ns²np². The inner core of the electronic configuration of elements in this group also differs.
Covalent Radius
There is a considerable increase in covalent radius from C to Si, thereafter from Si to Pb a small increase in radius is observed. This is due to the presence of completely filled d and f orbitals in heavier members.
Ionization Enthalpy
The first ionization enthalpy of group 14 members is higher than the corresponding members of group 13. The influence of inner core electrons is visible here also. In general, the ionisation enthalpy decreases down the group.
Electronegativity
Due to small size, the elements of this group are slightly more electronegative than group 13 elements. The electronegativity values for elements from Si to Pb are almost the same.
Physical Properties
All group 14 members are solids. Carbon and silicon are non-metals, germanium is a metalloid, whereas tin and lead are soft metal.
Chemical Properties Oxidation states and trends in chemical reactivity
The group 14 elements have four electrons in outermost shell. The common oxidation states exhibited by these elements are +4 and +2.
Carbon also exhibits negative oxidation states. Since the sum of the first four ionization enthalpies is very high, compounds in +4 oxidation state are generally covalent in nature. In heavier members the tendency to show +2 oxidation state increases in the sequence Ge<Sn (i) Reactivity towards oxygen
All members when heated in oxygen form oxides. There are mainly two types of oxides, monoxide, and dioxide of formula MO and MOs respectively.
(ii) Reactivity towards water
![]()
(iii) Reactivity towards halogen
These elements can form halides of formula MX2, and MX4 (where X = F, Cl, Br, I). Except carbon, all other members react directly with halogen under suitable condition to make halides.
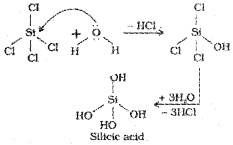
Hydrolysis can be understood by taking the example of SiCl4. It undergoes hydrolysis by initially accepting lone pair of electrons from water molecule in d orbitals of Si, finally leading to the formation of Si(OH)4 as shown below:
Important Trends And Anomalous Behaviour Of Carbon
Carbon differs from rest of the members of its group. It is due to its smaller size, higher electronegativity, higher ionisation enthalpy and unavailability of d orbitals. In carbon, only s and p orbitals are available for bonding and, therefore, it can accommodate only four pairs of electrons around it. This would limit the maximum covalence to four whereas other members can expand their covalence due to the presence of d orbitals.
Carbon has the ability to form pπ – pπ multiple bonds with itself and with other atoms of small size and high electronegativity.
Few examples are: C=C, C° C, C=0, C=S, and C° N. Carbon atoms have the tendency to link with one another through covalent bonds to form chains and rings. This property is called catenation.
Allotropes Of Carbon
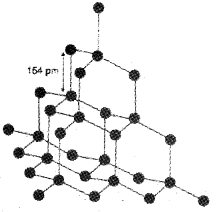
Diamond
It has a crystalline lattice. In diamond, each carbon atom undergoes sp³ hybridisation and linked to four other carbon atoms by using hybridised orbitals in tetrahedral fashion. The C-C bond length is 154 pm. In this structure, directional covalent bonds are present throughout the lattice. It is very difficult to break extended covalent bonding and, therefore, diamond is a hardest substance on the earth. It is used as an abrasive for sharpening hard tools.
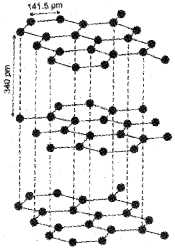
Graphite
Graphite has layered structure. Layers are held by van der Waals forces and distance between two layers is 340 pm. Each layer is composed of planar hexagonal rings of carbon atoms. C—C bond length within the layer is 141.5 pm. Each carbon atom in hexagonal ring undergoes sp² hybridisation and makes three sigma bonds with three neighbouring carbon atoms. Fourth electron forms a π bond. The electrons are delocalised over the whole sheet. Electrons are mobile and, therefore, graphite conducts electricity along the sheet. Graphite cleaves easily between the layers and, therefore, it is very soft and slippery. For this reason graphite is used as a dry lubricant in machines running at high temperature, where oil cannot be used as a lubricant.
Fullerenes
Fullerenes are prepared by heating graphite in an electric arc in the presence of helium or argon. The sooty material formed by condensation of the vapours consists of C60 with smaller amounts of C70 and other fullerenes. C60 is named as Buckminster fullerence. The general name fullerence refers to the family of spheroidal carbon-cage molecules. The shape of C60 resembles that of a soccer ball. It contains twelve five-membered rings and twenty 6-membered rings of carbon. The 6-membered rings are fused both to other five and six membered rings. However, the 5-membered rings are fused only to six-membered rings. Both carbon-carbon single (1.435 Å) and double (1.383 Å) bonds are present in this structure. Carbon black, coke and charcoal are impure amorphous forms of graphite or fullerenes. Carbon black is formed by burning hydrocarbon in limited supply of air. Charcoal and coke are obtained by heating wood and coal respectively in the absence of air.
Uses of Carbon
Being good conductor, graphite is used for electrodes in batteries and industrial electrolysis. Crucibles made from graphite are inert to dilute acids and alkalies. Being highly porous, activated charcoal is used in adsorbing poisonous gases. Diamond is a precious stone and used in jewellery.
Some Important Compounds Of Carbon And Silicon
Oxides of Carbon
Two important oxides of carbon are carbon monoxide, CO and carbon dioxide, CO2.
Carbon Monoxide
Direct oxidation of C in limited supply of oxygen or air yields carbon monoxide.
![]()
On commercial scale it is prepared by the passage of steam over hot coke. The mixture of CO and H2 thus produced is known as water gas or synthesis gas.
![]()
When air is used instead of steam, a mixture of CO and N2 is produced, which is called producer gas.
![]()
Water gas and producer gas are very important industrial fuels. Carbon monoxide in water gas or producer gas can undergo further combustion forming carbon dioxide with the liberation of heat. CO arises has the ability to form a complex with haemoglobin, which is about 300 times more stable than the oxygen-haemoglobin complex. This prevents haemoglobin in the red blood corpuscles from carrying oxygen round the body and ultimately resulting in death.
Carbon Dioxide
It is prepared by complete combustion of carbon and carbon-containing fuels in excess of air.
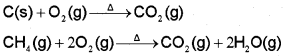
On commercial scale it is obtained by heating limestone. Carbon dioxide, which is normally present to the extent of ~0.03 % by volume in the atmosphere, is removed from it by the process known as photosynthesis. It is the process by which green plants convert atmospheric CO2 into carbohydrates such as glucose. The overall chemical change can be expressed as:
The increase in combustion of fossil fuels and decomposition of limestone for cement manufacture in recent years seem to increase the CO2 content of the atmosphere. This may lead to increase in green house effect and thus, raise the temperature of the atmosphere which might have serious consequences. Carbon dioxide can be obtained as a solid in the form of dry ice by allowing the liquified CO2 to expand rapidly. Dry ice is used as a refrigerant for ice-cream and frozen food.
![]()
Resonance structures of carbon dioxide
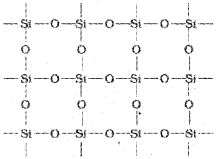
Silicon Dioxide, SiO2
Quartz, cristobatite and tridymite are some of the crystalline forms of silica, and they are interconvertible at suitable temperature. In Silicon dioxide, each silicon atom is covalently bonded in a tetrahedral manner to four oxygen atoms. Each oxygen atom in turn covalently bonded to another silicon atoms.
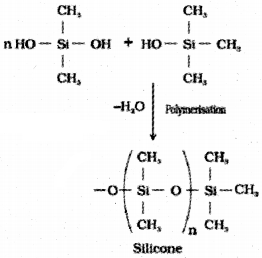
Silicones
They are a group of organosilicon polymers, which have (R2SiO) as a repeating unit. The starting materials for the manufacture of silicones are alkyl or aryl substituted silicon chlorides, RnSiCl(4-n), where R is alkyl or aryl group.
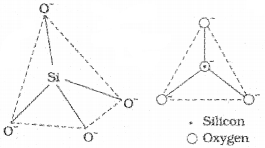
Silicates
The basic structural unit of silicates if SiO44- tertrahedra. Feldspar, zerolites, mica, asbestose, etc. are examples of silicates. In silicates, either the SiO44- will be present as discrete units or several such units are joined togetherth rough sharing of corner of the tetrahedra using one to four oxygen atoms per silicate unit. Like this, different silicates assume different forms such as chain, ring, sheet or three-dimensional structures. Glass and cement are examples of man-made silicates.
Zeolites
Zeolites are alumino silicates. If a few Si atoms of the three-dimensional network structure of SiO2 are replaced by Al atoms, the resulting structure is called alumino silicate structure. This structure evidently has negative charge and Na+.K+ pr Ca2+ ions balance the negative charge. Zeolites are used as catalysts in petrochemical industry for cracking of hydrocarbons. ZSM-5 is a type of zeolite used in the conversion of alcohol to gasoline. Zeolites are also used in softening hard water.
